The future of gene-targeted therapy for hereditary tyrosinemia type 1 as a lead indication among the inborn errors of metabolism
- PMID: 33224636
- PMCID: PMC7676758
- DOI: 10.1080/21678707.2020.1791082
The future of gene-targeted therapy for hereditary tyrosinemia type 1 as a lead indication among the inborn errors of metabolism
Abstract
Introduction: Inborn errors of metabolism (IEMs) often result from single-gene mutations and collectively cause liver dysfunction in neonates leading to chronic liver and systemic disease. Current treatments for many IEMs are limited to maintenance therapies that may still require orthotropic liver transplantation. Gene therapies offer a potentially superior approach by correcting or replacing defective genes with functional isoforms; however, they face unique challenges from complexities presented by individual diseases and their diverse etiology, presentation, and pathophysiology. Furthermore, immune responses, off-target gene disruption, and tumorigenesis are major concerns that need to be addressed before clinical application of gene therapy.
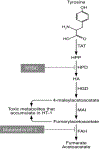
Areas covered: The current treatments for IEMs are reviewed as well as the advances in, and barriers to, gene therapy for IEMs. Attention is then given to ex vivo and in vivo gene therapy approaches for hereditary tyrosinemia type 1 (HT1). Of all IEMs, HT1 is particularly amenable to gene therapy because of a selective growth advantage conferred to corrected cells, thereby lowering the initial transduction threshold for phenotypic relevance.
Expert opinion: It is proposed that not only is HT1 a safe indication for gene therapy, its unique characteristics position it to be an ideal IEM to develop for clinical investigation.
Keywords: Adeno-associated viral vector; gene therapy; hereditary tyrosinemia type 1; inborn errors of metabolism; lentiviral vector.
Conflict of interest statement
Declaration of interest JBL is an inventor on a patent regarding gene therapy for metabolic diseases. RAK and JBL are founding board members of Cytotheryx, Inc., with affiliated ownership. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Reviewer disclosures Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Figures
Similar articles
-
Autologous Gene and Cell Therapy Provides Safe and Long-Term Curative Therapy in A Large Pig Model of Hereditary Tyrosinemia Type 1.Cell Transplant. 2019 Jan;28(1):79-88. doi: 10.1177/0963689718814188. Epub 2018 Nov 26. Cell Transplant. 2019. PMID: 30477316 Free PMC article.
-
Lentiviral Vector-mediated Gene Therapy of Hepatocytes Ex Vivo for Autologous Transplantation in Swine.J Vis Exp. 2018 Nov 4;(141):10.3791/58399. doi: 10.3791/58399. J Vis Exp. 2018. PMID: 30451238 Free PMC article.
-
Liver-directed gene-based therapies for inborn errors of metabolism.Expert Opin Biol Ther. 2021 Feb;21(2):229-240. doi: 10.1080/14712598.2020.1817375. Epub 2020 Oct 13. Expert Opin Biol Ther. 2021. PMID: 32880494 Review.
-
Biochemical and Clinical Aspects of Hereditary Tyrosinemia Type 1.Adv Exp Med Biol. 2017;959:9-21. doi: 10.1007/978-3-319-55780-9_2. Adv Exp Med Biol. 2017. PMID: 28755181 Review.
-
Inborn Errors of Metabolism Screening in Neonates: Current Perspective with Diagnosis and Therapy.Curr Pediatr Rev. 2022;18(4):274-285. doi: 10.2174/1573396318666220404194452. Curr Pediatr Rev. 2022. PMID: 35379134 Review.
Cited by
-
Diagnostic and Therapeutic Challenges of Hereditary Tyrosinemia Type 1 in Lebanon: A 12-Year Retrospective Review.Front Pediatr. 2021 Aug 6;9:698577. doi: 10.3389/fped.2021.698577. eCollection 2021. Front Pediatr. 2021. PMID: 34422723 Free PMC article.
-
Focusing on Ischemic Reperfusion Injury in the New Era of Dynamic Machine Perfusion in Liver Transplantation.Int J Mol Sci. 2024 Jan 17;25(2):1117. doi: 10.3390/ijms25021117. Int J Mol Sci. 2024. PMID: 38256190 Free PMC article. Review.
-
mRNA-based therapy proves superior to the standard of care for treating hereditary tyrosinemia 1 in a mouse model.Mol Ther Methods Clin Dev. 2022 Jul 15;26:294-308. doi: 10.1016/j.omtm.2022.07.006. eCollection 2022 Sep 8. Mol Ther Methods Clin Dev. 2022. PMID: 35949297 Free PMC article.
-
Progress in Gene Therapy for Hereditary Tyrosinemia Type 1.Pharmaceutics. 2025 Mar 18;17(3):387. doi: 10.3390/pharmaceutics17030387. Pharmaceutics. 2025. PMID: 40143050 Free PMC article. Review.
-
Hereditary tyrosinemia type Ⅰ: newborn screening, diagnosis and treatment.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021 Aug 25;50(4):514-523. doi: 10.3724/zdxbyxb-2021-0255. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021. PMID: 34704422 Free PMC article. English.
References
-
- Hansen K, Horslen S. Metabolic liver disease in children.Liver Transpl. 2008. [May];14(5):713–733. - PubMed
-
- Hegarty R, Hadzic N, Gissen P, et al. Inherited metabolic disorders presenting as acute liver failure in newborns and young children: King’s college hospital experience. Eur J Pediatr. 2015. [October];174 (10):1387–1392. - PubMed
-
- Zeybek AC, Kiykim E, Soyucen E, et al. Hereditary tyrosinemia type 1 in Turkey: twenty year single-center experience. Pediatr Int. 2015. [April];57(2):281–289. - PubMed
-
- Masurel-Paulet A, Poggi-Bach J, Rolland MO, et al. NTBC treatment in tyrosinaemia type I: long-term outcome in French patients. J Inherit Metab Dis. 2008. [February];31(1):81–87. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources