Optical coherence tomography: fundamentals and clinical utility
- PMID: 33224764
- PMCID: PMC7666937
- DOI: 10.21037/cdt-20-253
Optical coherence tomography: fundamentals and clinical utility
Abstract
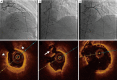
Although coronary angiography is the standard method employed to assess the severity of coronary artery disease and to guide treatment strategies, it provides only 2D image of the intravascular lesions. In contrast, intravascular imaging modalities such as optical coherence tomography (OCT) produce cross-sectional images of the coronary arteries at a far greater spatial resolution, capable of accurately determining vessel size as well as plaque morphology, eliminating many of the disadvantages inherent to angiography. This review will discuss the role of OCT in the catherization laboratory for the assessment and management of coronary disease.
Keywords: Acute coronary syndromes (ACS); coronary artery disease (CAD); tomography, optical coherence.
2020 Cardiovascular Diagnosis and Therapy. All rights reserved.
Conflict of interest statement
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure forms (available at http://dx.doi.org/10.21037/cdt-20-253). The series “Intracoronary Imaging” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Figures








References
-
- Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol 2012;59:1058-72. 10.1016/j.jacc.2011.09.079 - DOI - PubMed
-
- Jang IK. Cardiovascular OCT Imaging. 2nd edition. Springer, 2020.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
