Left ventricular dysfunction in heart failure with preserved ejection fraction-molecular mechanisms and impact on right ventricular function
- PMID: 33224773
- PMCID: PMC7666919
- DOI: 10.21037/cdt-20-477
Left ventricular dysfunction in heart failure with preserved ejection fraction-molecular mechanisms and impact on right ventricular function
Abstract
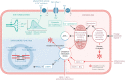
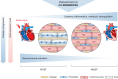
The current classification of heart failure (HF) based on left ventricular (LV) ejection fraction (EF) identifies a large group of patients with preserved ejection fraction (HFpEF) with significant morbidity and mortality but without prognostic benefit from current HF therapy. Co-morbidities and conditions such as arterial hypertension, diabetes mellitus, chronic kidney disease, adiposity and aging shape the clinical phenotype and contribute to mortality. LV diastolic dysfunction and LV structural remodeling are hallmarks of HFpEF, and are linked to remodeling of the cardiomyocyte and extracellular matrix. Pulmonary hypertension (PH) and right ventricular dysfunction (RVD) are particularly common in HFpEF, and mortality is up to 10-fold higher in HFpEF patients with vs. without RV dysfunction. Here, we review alterations in cardiomyocyte function (i.e., ion homeostasis, sarcomere function and cellular metabolism) associated with diastolic dysfunction and summarize the main underlying cellular pathways. The contribution and interaction of systemic and regional upstream signaling such as chronic inflammation, neurohumoral activation, and NO-cGMP-related pathways are outlined in detail, and their diagnostic and therapeutic potential is discussed in the context of preclinical and clinical studies. In addition, we summarize prevalence and pathomechanisms of RV dysfunction in the context of HFpEF and discuss mechanisms connecting LV and RV dysfunction in HFpEF. Dissecting the molecular mechanisms of LV and RV dysfunction in HFpEF may provide a basis for an improved classification of HFpEF and for therapeutic approaches tailored to the molecular phenotype.
Keywords: Heart failure (HF); calcium; cardiomyocytes; metabolism; pulmonary hypertension (PH); right ventricle; sodium.
2020 Cardiovascular Diagnosis and Therapy. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-477). The series “Right Ventricular Dysfunction” was commissioned by the editorial office without any funding or sponsorship. Dr. RA de Boer reports grants from Abbott, grants from AstraZeneca, grants from Bristol-Myers Squibb, grants from Novartis, grants from NovoNordisk, grants from Roche, personal fees from Abbott, personal fees from AstraZeneca, personal fees from NovoNordisk, personal fees from Roche, outside the submitted work. Dr. WMK reports grants from Heart and Stroke Foundation of Canada, grants from German Center for Cardiovascular Research, grants from German Research Foundation, grants from German Ministry for Education and Research, during the conduct of the study. The authors have no other conflicts of interest to declare.
Figures

References
-
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. 10.1093/eurheartj/ehw128 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
