Right ventricular dysfunction and long-term risk of death
- PMID: 33224778
- PMCID: PMC7666957
- DOI: 10.21037/cdt-20-450
Right ventricular dysfunction and long-term risk of death
Abstract
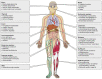
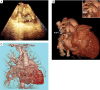
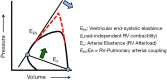
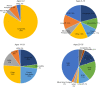
Sudden cardiac death (SCD), or sudden loss of life-sustaining systemic and cerebral perfusion, is most often due to left ventricular (LV) dysfunction secondary to ischemic or structural cardiac disease or channelopathies. Degeneration of sinus rhythm into ventricular tachycardia and ultimately ventricular fibrillation is the final common pathway for most heart failure patients. Right ventricular (RV) dysfunction is recognized as an independent contributor to worsening heart failure. There is emerging evidence that RV dysfunction may also be an independent predictor of SCD. This review examines the role of RV dysfunction on modifying long term risk of SCD, and explores possible mechanisms that may underlie SCD. The RV has unique anatomy and physiology compared to the LV. Subsequently, we begin with a review of cardiac embryology, focusing on the chambers, valves, coronary arteries, and cardiac conduction system to understand the origins of RV dysfunction. Static and dynamic physiology of the RV is contrasted with that of the LV. Particular emphasis is placed on ventriculo-arterial coupling, mechanical cardiac constraint, and ventricular interdependence. The epidemiology of SCD is briefly reviewed to highlight how causes of SCD are age-specific. In turn, the age-specific causes of RV dysfunction are presented, including those which predominate in childhood and adolescence [arrhythmogenic RV dysplasia (ARVD) and hypertrophic cardiomyopathy (HCM)] and older adulthood (cardiac ischemia, chronic congestive heart failure and post-capillary pulmonary hypertension, and pulmonary hypertension). There is a clear need for additional studies on the independent contribution of RV dysfunction to overall functional capacity, SCD-associated mortality, and non-SCD-associated mortality. Discovery would be aided by the development of prospective cohorts with excellent RV phenotyping, coupled with deeper biologic measurements linking mechanisms to clinically relevant outcomes.
Keywords: Right ventricle; arrhythmogenic right ventricular dysplasia (ARVD); hypertrophic cardiomyopathy (HCM); pulmonary hypertension; sudden cardiac death (SCD).
2020 Cardiovascular Diagnosis and Therapy. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-450). The series “Right Ventricular Dysfunction” was commissioned by the editorial office without any funding or sponsorship. Dr. MK served as the unpaid Guest Editors of the series. Dr. SR reports personal fees and other from Abbott, grants and personal fees from Actelion, personal fees from Acceleron, grants and personal fees from AstraZeneca, grants and personal fees from Bayer, personal fees from BMS, personal fees from Janssen, personal fees from Merck, grants and personal fees from Novartis, grants and personal fees from Pfizer, grants and personal fees from United Therapeutics, personal fees from Vifor, outside the submitted work. Dr. BAM reports other from Acetelion, during the conduct of the study. The author has no other conflicts of interest to declare.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
