Safety and efficacy of the endothelin receptor antagonist macitentan in pediatric pulmonary hypertension
- PMID: 33224780
- PMCID: PMC7666951
- DOI: 10.21037/cdt.2020.04.01
Safety and efficacy of the endothelin receptor antagonist macitentan in pediatric pulmonary hypertension
Abstract
Background: Macitentan, a dual endothelin receptor antagonist (ERA), was approved in 2014 for the treatment of adults with idiopathic pulmonary arterial hypertension (PAH). Once-per-day dosing and low potential hepatic toxicity make macitentan an appealing therapeutic option for children with PAH, but reports on its use in pediatric patients are still lacking.
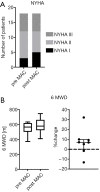
Methods: Prospective observational study of 18 children [10 male; median age: 8.5, minimum (min.): 0.6, maximum (max.): 16.8 years] with pulmonary hypertension (PH). Four of these 18 patients were treatment-naïve and started on a de novo macitentan therapy. The remaining 14/18 children were already on a PH-targeted pharmacotherapy (sildenafil or bosentan as monotherapy or in combination). Nine children who were on bosentan were switched to macitentan. We analyzed the 6-minute walking distance (6MWD), NYHA functional class (FC)/modified ROSS score, invasive hemodynamics, echocardiographic variables and the biomarker N-terminal pro-brain natriuretic peptide (NT-proBNP).
Results: The median follow up was 6 months (min.: 0.5, max.: 30). Macitentan treatment was associated with improvement of invasive hemodynamics, e.g., the ratio of mean pulmonary arterial pressure/mean systemic arterial pressure decreased from a median of 62% (min.: 30%, max.: 87%) to 49% (min.: 30%, max.: 69%), P<0.05; pulmonary vascular resistance index (PVRi) decreased from a median of 7.6 (min.: 3.3, max.: 11.5) to 4.8 Wood units × m2 body surface area (min.: 2.5, max.: 10), P<0.05. The tricuspid annular plane systolic excursion (TAPSE) increased from a median of 1.4 (min.: 0.8, max.: 2.8) to 1.9 (min.: 0.8, max.: 2.7) cm, (P<0.05). NT-proBNP values decreased from a median of 272 (min.: 27, max.: 2,010) to 229 (min.: 23, max.: 814) pg/mL under macitentan therapy (P<0.05). The 6MWD and NYHA FC/modified ROSS score did not change significantly.
Conclusions: This is the first prospective study of macitentan pharmacotherapy in infants and children with PH <12 years of age. Except in one patient, macitentan treatment was well tolerated and was associated with improvements in invasive hemodynamics, longitudinal systolic RV function (TAPSE) and serum NT-proBNP values.
Keywords: Bosentan; child; endothelin receptor antagonist (ERA); macitentan; pulmonary hypertension (PH).
2020 Cardiovascular Diagnosis and Therapy. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt.2020.04.01). The series “Right Ventricular Dysfunction” was commissioned by the editorial office without any funding or sponsorship. AG reports grants from JNJ during the conduct of the study. Actelion/Johnson&Johnson had no influence on this publication, has not seen the data or the manuscript and had no role in the production of this manuscript. The authors are solely responsible for the design and conduction of this study, all study analyses, the drafting and editing of the paper and its final contents. The authors have no other conflicts of interest to declare.
Figures




Similar articles
-
Efficacy and safety of switching from bosentan or ambrisentan to macitentan in pulmonary arterial hypertension: A systematic review and meta-analysis.Front Cardiovasc Med. 2022 Dec 7;9:977110. doi: 10.3389/fcvm.2022.977110. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36568539 Free PMC article.
-
Clinical use of macitentan in the treatment of connective tissue disease-associated pulmonary arterial hypertension.J Thorac Dis. 2024 Mar 29;16(3):2060-2069. doi: 10.21037/jtd-24-151. Epub 2024 Mar 27. J Thorac Dis. 2024. PMID: 38617769 Free PMC article.
-
[Dynamics of the clinical functional and hemodynamic profile of patients with pulmonary arterial hypertension with initial monotherapy with endothelin receptor antagonists: bosentan vs. macitentan].Kardiologiia. 2020 Aug 11;60(7):28-35. doi: 10.18087/cardio.2020.7.n1136. Kardiologiia. 2020. PMID: 33155938 Russian.
-
The clinical experience of macitentan in pulmonary hypertension in Indian cohort: 12-month follow-up.Lung India. 2022 Jan-Feb;39(1):12-15. doi: 10.4103/lungindia.lungindia_671_20. Lung India. 2022. PMID: 34975047 Free PMC article.
-
A Focus on Macitentan in the Treatment of Pulmonary Arterial Hypertension.Basic Clin Pharmacol Toxicol. 2018 Aug;123(2):103-113. doi: 10.1111/bcpt.13033. Epub 2018 Jun 5. Basic Clin Pharmacol Toxicol. 2018. PMID: 29719121 Review.
Cited by
-
Efficacy and safety of switching from bosentan or ambrisentan to macitentan in pulmonary arterial hypertension: A systematic review and meta-analysis.Front Cardiovasc Med. 2022 Dec 7;9:977110. doi: 10.3389/fcvm.2022.977110. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36568539 Free PMC article.
-
Getting to the bottom of right heart failure.Cardiovasc Diagn Ther. 2020 Oct;10(5):1517-1521. doi: 10.21037/cdt-20-565. Cardiovasc Diagn Ther. 2020. PMID: 33224771 Free PMC article. No abstract available.
-
Safety, tolerability, and efficacy of an in-class combination therapy switch from bosentan plus sildenafil to ambrisentan plus tadalafil in children with pulmonary arterial hypertension.Pulm Circ. 2024 Dec 26;14(4):e70011. doi: 10.1002/pul2.70011. eCollection 2024 Oct. Pulm Circ. 2024. PMID: 39734932 Free PMC article.
-
Pulmonary hypertension in bronchopulmonary dysplasia.Pediatr Res. 2021 Feb;89(3):446-455. doi: 10.1038/s41390-020-0993-4. Epub 2020 Jun 10. Pediatr Res. 2021. PMID: 32521539 Free PMC article. Review.
-
Macitentan in the Young-Mid-term Outcomes of Patients with Pulmonary Hypertensive Vascular Disease treated in a Pediatric Tertiary Care Center.Paediatr Drugs. 2023 Jul;25(4):467-481. doi: 10.1007/s40272-023-00573-y. Epub 2023 Jun 3. Paediatr Drugs. 2023. PMID: 37269500 Free PMC article.
References
-
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. 10.1093/eurheartj/ehv317 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
