Alternol/Alteronol: Potent Anti-cancer Compounds With Multiple Mechanistic Actions
- PMID: 33224877
- PMCID: PMC7670956
- DOI: 10.3389/fonc.2020.568110
Alternol/Alteronol: Potent Anti-cancer Compounds With Multiple Mechanistic Actions
Abstract
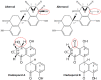
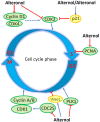
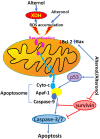
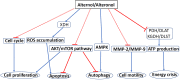
Alternol and its oxidate isomer Alteronol are small compounds isolated from the fermentation of a mutant fungus obtained from Taxus brevifolia bark. Preclinical studies showed their potent anti-cancer activities, including attenuating cellular survival pathways, altering protein levels of cell cycle regulators, activating xanthine dehydrogenase to cause accumulation of cellular reactive oxygen species and disrupting cell metabolism by disturbing four Krebs cycle enzymes specifically in malignant cells while having no significant effect on benign cells. In cancer cell culture models, Alternol or Alteronol exert their anti-cancer effect by inducing cell cycle arrest and triggering apoptotic cell death. In mice xenograft models, Alternol or Alteronol potently suppresses tumor growth with no obvious toxicity to the host with a wide therapeutic index over 30-fold. In conclusion, Alternol or Alteronol possess a great potential and feasibility to be developed as an effective anti-tumor therapeutic.
Keywords: Alternol; Alteronol; Cladosporol; apoptosis; cell cycle; radical oxygen species.
Copyright © 2020 Liu, Li, Huang, Chen, Holzbeierlein and Li.
Figures
Similar articles
-
Natural compound Alternol exerts a broad anti-cancer spectrum and a superior therapeutic safety index in vivo.Front Pharmacol. 2024 May 24;15:1409506. doi: 10.3389/fphar.2024.1409506. eCollection 2024. Front Pharmacol. 2024. PMID: 38855749 Free PMC article.
-
Natural compound Alternol actives multiple endoplasmic reticulum stress-responding pathways contributing to cell death.Front Pharmacol. 2024 May 20;15:1397116. doi: 10.3389/fphar.2024.1397116. eCollection 2024. Front Pharmacol. 2024. PMID: 38831880 Free PMC article.
-
Alternol eliminates excessive ATP production by disturbing Krebs cycle in prostate cancer.Prostate. 2019 May;79(6):628-639. doi: 10.1002/pros.23767. Epub 2019 Jan 20. Prostate. 2019. PMID: 30663084 Free PMC article.
-
Natural compound Alternol as a novel therapeutic for prostate cancer treatment.Am J Clin Exp Urol. 2020 Jun 25;8(3):76-80. eCollection 2020. Am J Clin Exp Urol. 2020. PMID: 32699806 Free PMC article. Review.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
Cited by
-
Natural compound Alternol exerts a broad anti-cancer spectrum and a superior therapeutic safety index in vivo.Front Pharmacol. 2024 May 24;15:1409506. doi: 10.3389/fphar.2024.1409506. eCollection 2024. Front Pharmacol. 2024. PMID: 38855749 Free PMC article.
-
Alternol Sensitizes Renal Carcinoma Cells to TRAIL-Induced Apoptosis.Front Pharmacol. 2021 Mar 25;12:560903. doi: 10.3389/fphar.2021.560903. eCollection 2021. Front Pharmacol. 2021. PMID: 33841136 Free PMC article.
-
Natural compound Alternol actives multiple endoplasmic reticulum stress-responding pathways contributing to cell death.Front Pharmacol. 2024 May 20;15:1397116. doi: 10.3389/fphar.2024.1397116. eCollection 2024. Front Pharmacol. 2024. PMID: 38831880 Free PMC article.
References
-
- Duan L, Chen H, Chen J, Hong H, Li W. The fermentation process of the new anticancer compound Alternol. J Shenyang Pharm Univ. (2009) 26:316–23.
-
- Cheng S, Wang R, Chen J, Duan L, Qiu X. Isolation, purification and identification of paclitaxel from the fermentated extra of fungus ST026 (Alternaria alternate var.monosporus). J Chin Pharm Univ. (2011) 42:570–2.
-
- Duan L, Chen HR, Chen JP, Li WP, Hong L. Screening for the high-yield paclitaxel producing strain Alternaria alternate var.monosporus. Chinese J Antibiotics. (2008) 34:650–2.
-
- Chen J, Duan LL, Chen HR, Lin H, Li WP, Luo JG, et al. Studies on the antifungal activities of Alterfungin and its derivatives. Chin J Antibiotics. (2009) 34:15–7.
Publication types
LinkOut - more resources
Full Text Sources

