The Role of Small Extracellular Vesicles in Viral-Protozoan Symbiosis: Lessons From Trichomonasvirus in an Isogenic Host Parasite Model
- PMID: 33224901
- PMCID: PMC7674494
- DOI: 10.3389/fcimb.2020.591172
The Role of Small Extracellular Vesicles in Viral-Protozoan Symbiosis: Lessons From Trichomonasvirus in an Isogenic Host Parasite Model
Abstract
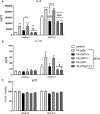
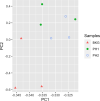
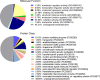
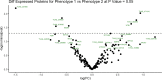
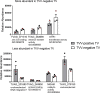
The protozoan parasite Trichomonas vaginalis (TV), exclusively adapted to the human genital tract, is one of the most common sexually transmitted pathogens. Adding to the complexity of the host-pathogen interactions, the parasite harbors TV-specific endosymbiont viruses (Trichomonasvirus, TVV). It was reported that small extracellular vesicles (sEVs) released by TV play a role in host immunity; however, the role of the viral endosymbiosis in this process remained unknown. We hypothesized that the virus may offer evolutionary benefit to its protozoan host at least in part by altering the immunomodulatory properties of sEVs spreading from the site of infection to non-infected immune effector cells. We infected human vaginal epithelial cells, the natural host of the parasite, with TV natively harboring TVV and an isogenic derivative of the parasite cured from the viral infection. sEVs were isolated from vaginal cell culture 24 h post TV infection and from medium where the isogenic TV strains were cultured in the absence of the human host. sEVs from TVV-negative but not TVV-positive parasites cultured alone caused NF-κB activation and increase of IL-8 and RANTES expression by uterine endocervical cells, which provide innate immune defense at the gate to the upper reproductive tract. Similarly, mononuclear leukocytes increased their IL-8, IL-6 and TNF-α output in response to sEVs from virus-negative, but not isogenic virus-positive parasites, the latter exosomes being immunosuppressive in comparison to TV medium control. The same phenomenon of suppressed immunity induced by the TVV-positive compared to TVV-negative phenotype was seen when stimulating the leukocytes with sEVs originating from infected vaginal cultures. In addition, the sEVs from the TVV-positive infection phenotype suppressed immune signaling of a toll-like receptor ligand derived from mycoplasma, another frequent TV symbiont. Quantitative comparative proteome analysis of the secreted sEVs from virus-positive versus virus-negative TV revealed differential expression of two functionally uncharacterized proteins and five proteins involved in Zn binding, protein binding, electron transfer, transferase and catalytic activities. These data support the concept that symbiosis with viruses may provide benefit to the protozoan parasite by exploiting sEVs as a vehicle for inter-cellular communications and modifying their protein cargo to suppress host immune activation.
Keywords: T. vaginalis; Trichomonasvirus; cytokines; exosomes; extracellular vesicles; immune modulation; proteomics.
Copyright © 2020 Govender, Chan, Yamamoto, Budnik and Fichorova.
Figures

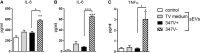
Similar articles
-
Double-Stranded RNA Viruses Are Released From Trichomonas vaginalis Inside Small Extracellular Vesicles and Modulate the Exosomal Cargo.Front Microbiol. 2022 May 4;13:893692. doi: 10.3389/fmicb.2022.893692. eCollection 2022. Front Microbiol. 2022. PMID: 35602021 Free PMC article.
-
Identification of Endosymbiotic Virus in Small Extracellular Vesicles Derived from Trichomonas vaginalis.Genes (Basel). 2022 Mar 17;13(3):531. doi: 10.3390/genes13030531. Genes (Basel). 2022. PMID: 35328084 Free PMC article.
-
Cytidine nucleoside analog is an effective antiviral drug against Trichomonasvirus.J Microbiol Immunol Infect. 2022 Apr;55(2):191-198. doi: 10.1016/j.jmii.2021.08.008. Epub 2021 Aug 25. J Microbiol Immunol Infect. 2022. PMID: 34479802
-
Trichomonas vaginalis Virus: Current Insights and Emerging Perspectives.Viruses. 2025 Jun 26;17(7):898. doi: 10.3390/v17070898. Viruses. 2025. PMID: 40733518 Free PMC article. Review.
-
Neutrophil interactions with the sexually transmitted parasite Trichomonas vaginalis: implications for immunity and pathogenesis.Open Biol. 2020 Sep;10(9):200192. doi: 10.1098/rsob.200192. Epub 2020 Sep 2. Open Biol. 2020. PMID: 32873151 Free PMC article. Review.
Cited by
-
Recent advances in the molecular biology of the protist parasite Trichomonas vaginalis.Fac Rev. 2021 Mar 4;10:26. doi: 10.12703/r/10-26. eCollection 2021. Fac Rev. 2021. PMID: 33718943 Free PMC article. Review.
-
Multiple Regulations of Parasitic Protozoan Viruses: A Double-Edged Sword for Protozoa.mBio. 2023 Feb 28;14(1):e0264222. doi: 10.1128/mbio.02642-22. Epub 2023 Jan 12. mBio. 2023. PMID: 36633419 Free PMC article. Review.
-
Distinct Microbial Communities in Dilated Cardiomyopathy Explanted Hearts Are Associated With Different Myocardial Rejection Outcomes.Front Cell Infect Microbiol. 2021 Nov 29;11:732276. doi: 10.3389/fcimb.2021.732276. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34912727 Free PMC article.
-
Extracellular Vesicles and Their Impact on the Biology of Protozoan Parasites.Trop Med Infect Dis. 2023 Sep 15;8(9):448. doi: 10.3390/tropicalmed8090448. Trop Med Infect Dis. 2023. PMID: 37755909 Free PMC article. Review.
-
Double-Stranded RNA Viruses Are Released From Trichomonas vaginalis Inside Small Extracellular Vesicles and Modulate the Exosomal Cargo.Front Microbiol. 2022 May 4;13:893692. doi: 10.3389/fmicb.2022.893692. eCollection 2022. Front Microbiol. 2022. PMID: 35602021 Free PMC article.
References
-
- Aurrecoechea C., Brestelli J., Brunk B. P., Carlton J. M., Dommer J., Fischer S., et al. (2009). GiardiaDB and TrichDB: integrated genomic resources for the eukaryotic protist pathogens Giardia lamblia and Trichomonas vaginalis. Nucleic Acids Res. 37, D526–D530. 10.1093/nar/gkn631 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

