Neuronal megalin mediates synaptic plasticity-a novel mechanism underlying intellectual disabilities in megalin gene pathologies
- PMID: 33225275
- PMCID: PMC7667529
- DOI: 10.1093/braincomms/fcaa135
Neuronal megalin mediates synaptic plasticity-a novel mechanism underlying intellectual disabilities in megalin gene pathologies
Abstract
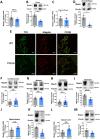
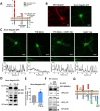
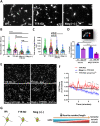
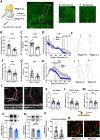
Donnai-Barrow syndrome, a genetic disorder associated to LRP2 (low-density lipoprotein receptor 2/megalin) mutations, is characterized by unexplained neurological symptoms and intellectual deficits. Megalin is a multifunctional endocytic clearance cell-surface receptor, mostly described in epithelial cells. This receptor is also expressed in the CNS, mainly in neurons, being involved in neurite outgrowth and neuroprotective mechanisms. Yet, the mechanisms involved in the regulation of megalin in the CNS are poorly understood. Using transthyretin knockout mice, a megalin ligand, we found that transthyretin positively regulates neuronal megalin levels in different CNS areas, particularly in the hippocampus. Transthyretin is even able to rescue megalin downregulation in transthyretin knockout hippocampal neuronal cultures, in a positive feedback mechanism via megalin. Importantly, transthyretin activates a regulated intracellular proteolysis mechanism of neuronal megalin, producing an intracellular domain, which is translocated to the nucleus, unveiling megalin C-terminal as a potential transcription factor, able to regulate gene expression. We unveil that neuronal megalin reduction affects physiological neuronal activity, leading to decreased neurite number, length and branching, and increasing neuronal susceptibility to a toxic insult. Finally, we unravel a new unexpected role of megalin in synaptic plasticity, by promoting the formation and maturation of dendritic spines, and contributing for the establishment of active synapses, both in in vitro and in vivo hippocampal neurons. Moreover, these structural and synaptic roles of megalin impact on learning and memory mechanisms, since megalin heterozygous mice show hippocampal-related memory and learning deficits in several behaviour tests. Altogether, we unveil a complete novel role of megalin in the physiological neuronal activity, mainly in synaptic plasticity with impact in learning and memory. Importantly, we contribute to disclose the molecular mechanisms underlying the cognitive and intellectual disabilities related to megalin gene pathologies.
Keywords: Donnai-Barrow syndrome; hippocampus; learning and memory; megalin; synaptic plasticity.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures





Similar articles
-
Transthyretin provides trophic support via megalin by promoting neurite outgrowth and neuroprotection in cerebral ischemia.Cell Death Differ. 2016 Nov 1;23(11):1749-1764. doi: 10.1038/cdd.2016.64. Epub 2016 Aug 12. Cell Death Differ. 2016. PMID: 27518433 Free PMC article.
-
Functional Consequences of Synapse Remodeling Following Astrocyte-Specific Regulation of Ephrin-B1 in the Adult Hippocampus.J Neurosci. 2018 Jun 20;38(25):5710-5726. doi: 10.1523/JNEUROSCI.3618-17.2018. Epub 2018 May 23. J Neurosci. 2018. PMID: 29793972 Free PMC article.
-
Fibroblast growth factor 23 signaling in hippocampal cells: impact on neuronal morphology and synaptic density.J Neurochem. 2016 Jun;137(5):756-69. doi: 10.1111/jnc.13585. Epub 2016 Mar 7. J Neurochem. 2016. PMID: 26896818
-
Nr4a2 Transcription Factor in Hippocampal Synaptic Plasticity, Memory and Cognitive Dysfunction: A Perspective Review.Front Mol Neurosci. 2021 Nov 22;14:786226. doi: 10.3389/fnmol.2021.786226. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34880728 Free PMC article. Review.
-
New insights into the roles of megalin/LRP2 and the regulation of its functional expression.Biol Res. 2011;44(1):89-105. doi: 10.4067/S0716-97602011000100012. Epub 2011 May 11. Biol Res. 2011. PMID: 21720686 Review.
Cited by
-
The role of transthyretin in cell biology: impact on human pathophysiology.Cell Mol Life Sci. 2021 Sep;78(17-18):6105-6117. doi: 10.1007/s00018-021-03899-3. Epub 2021 Jul 23. Cell Mol Life Sci. 2021. PMID: 34297165 Free PMC article. Review.
-
Identification of ecdysone receptor target genes in the worker honey bee brains during foraging behavior.Sci Rep. 2023 Jun 28;13(1):10491. doi: 10.1038/s41598-023-37001-7. Sci Rep. 2023. PMID: 37380789 Free PMC article.
-
Metallothioneins and Megalin Expression Profiling in Premalignant and Malignant Oral Squamous Epithelial Lesions.Cancers (Basel). 2021 Sep 9;13(18):4530. doi: 10.3390/cancers13184530. Cancers (Basel). 2021. PMID: 34572758 Free PMC article.
-
The role of lipocalin 2 in brain injury and recovery after ischemic and hemorrhagic stroke.Front Mol Neurosci. 2022 Sep 15;15:930526. doi: 10.3389/fnmol.2022.930526. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36187347 Free PMC article. Review.
-
Transcriptomic Analysis of Long Non-coding RNA-MicroRNA-mRNA Interactions in the Nucleus Accumbens Related to Morphine Addiction in Mice.Front Psychiatry. 2022 Jun 2;13:915398. doi: 10.3389/fpsyt.2022.915398. eCollection 2022. Front Psychiatry. 2022. PMID: 35722589 Free PMC article.
References
-
- Almeida MR, Damas AM, Lans MC, Brouwer A, Saraiva MJ. Thyroxine binding to transthyretin Met 119. Comparative studies of different heterozygotic carriers and structural analysis. Endocrine 1997; 6: 309–15. - PubMed
-
- Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, et al. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ 2005; 12: 1329–43. - PubMed
-
- Alvira-Botero X, Perez-Gonzalez R, Spuch C, Vargas T, Antequera D, Garzon M, et al. Megalin interacts with APP and the intracellular adapter protein FE65 in neurons. Mol Cell Neurosci 2010; 45: 306–15. - PubMed
-
- Ambjorn M, Asmussen JW, Lindstam M, Gotfryd K, Jacobsen C, Kiselyov VV, et al. Metallothionein and a peptide modeled after metallothionein, EmtinB, induce neuronal differentiation and survival through binding to receptors of the low-density lipoprotein receptor family. J Neurochem 2008; 104: 21–37. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
