Pyridinic Nanographenes by Novel Precursor Design
- PMID: 33225488
- PMCID: PMC7898602
- DOI: 10.1002/chem.202004983
Pyridinic Nanographenes by Novel Precursor Design
Abstract
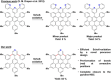
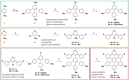
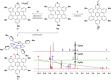
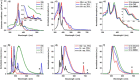
In this work we present the solution-synthesis of pyridine analogues to hexa-peri-hexabenzocoronene (HBC)-which might be called superpyridines-via a novel precursor design. The key step in our strategy was the pre-formation of the C-C bonds between the 3/3' positions of the pyridine and the adjacent phenyl rings-bonds that are otherwise unreactive and difficult to close under Scholl-conditions. Apart from the synthesis of the parent compound we show that classical pyridine chemistry, namely oxidation, N-alkylation and metal-coordination is applicable to the π-extended analogue. Furthermore, we present basic physical chemical characterizations of the newly synthesized molecules. With this novel synthetic strategy, we hope to unlock the pyridine chemistry of nanographenes.
Keywords: Scholl oxidation; heterocycles; nanographene; polycyclic aromatic hydrocarbons; pyridine.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

