Amphiphilic Polyelectrolyte Graft Copolymers Enhance the Activity of Cyclic Dinucleotide STING Agonists
- PMID: 33225632
- PMCID: PMC7856189
- DOI: 10.1002/adhm.202001056
Amphiphilic Polyelectrolyte Graft Copolymers Enhance the Activity of Cyclic Dinucleotide STING Agonists
Abstract
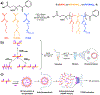
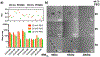
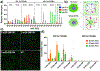
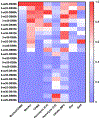
Cyclic dinucleotide (CDN) agonists of stimulator of interferon genes (STING) hold great therapeutic potential, but their activity is hindered by poor drug-like properties that restrict cytosolic bioavailability. Here, this challenge is addressed through the synthesis and evaluation of a novel series of PEGMA-co-DEAEMA-co-BMA copolymers with pH-responsive, membrane-destabilizing activity to enhance intracellular delivery of the CDN, cGAMP. Copolymers are synthesized with PEGMA of two different molecular weights (300 and 950 Da) and over a range of PEG mass fraction and polymer molecular weight, and relationships between copolymer structure, self-assembly, endosomal escape, and cGAMP activity are elucidated. A subset of polymers that self-assembled into 50-800 nm nanoparticles is identified, which can be loaded with cGAMP via a simple mixing strategy, resulting in significantly enhanced immunostimulatory activity. Increased cGAMP activity is found to be highly correlated with the capacity of carriers to enhance intracellular CDN uptake and to promote endosomal destabilization, findings that establish efficient cytosolic delivery as a criterion for CDN carriers. Additionally, it is demonstrated that a lead CDN carrier formulation can enhance STING activation in vivo in a model of intratumoral immunotherapy. Collectively, these investigations demonstrate the utility of PEGMA-co-DEAEMA-co-BMA copolymers as carriers for CDNs and potentially other cytosolically-acting drug cargo.
Keywords: endosomal escape; graft copolymers; polymer nanoparticles; self-assembling nanoparticles; stimulator of interferon genes (STING).
© 2020 Wiley-VCH GmbH.
Figures

References
-
- Flood BA, Higgs EF, Li S, Luke JJ, Gajewski TF, Immunol Rev 2019, 290, 24; - PMC - PubMed
- Li X-D, Wu J, Gao D, Wang H, Sun L, Chen ZJ, Science 2013, 341, 1390; - PMC - PubMed
- Dubensky TW Jr., Kanne DB, Leong ML, Ther Adv Vaccines 2013, 1, 131; - PMC - PubMed
- Gutjahr A, Papagno L, Nicoli F, Kanuma T, Kuse N, Cabral-Piccin MP, Rochereau N, Gostick E, Lioux T, Perouzel E, Price DA, Takiguchi M, Verrier B, Yamamoto T, Paul S, Appay V, JCI Insight 2019, 4. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

