Stimuli-Responsive Iron Oxide Nanotheranostics: A Versatile and Powerful Approach for Cancer Therapy
- PMID: 33225633
- PMCID: PMC7933107
- DOI: 10.1002/adhm.202001044
Stimuli-Responsive Iron Oxide Nanotheranostics: A Versatile and Powerful Approach for Cancer Therapy
Abstract
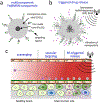
Recent advancements in unravelling elements of cancer biology involved in disease progression and treatment resistance have highlighted the need for a holistic approach to effectively tackle cancer. Stimuli-responsive nanotheranostics based on iron oxide nanoparticles are an emerging class of versatile nanomedicines with powerful capabilities to "seek, sense, and attack" multiple components of solid tumors. In this work, the rationale for using iron oxide nanoparticles and the basic physical principles that impact their function in biomedical applications are reviewed. Subsequently, recent advances in the integration of iron oxide nanoparticles with various stimulus mechanisms to facilitate the development of stimuli-responsive nanotheranostics for application in cancer therapy are summarized. The integration of an iron oxide core with various surface coating mechanisms results in the generation of hybrid nanoconstructs with capabilities to codeliver a wide variety of highly potent anticancer therapeutics and immune modulators. Finally, emerging future directions and considerations for their clinical translation are touched upon.
Keywords: cancer; iron oxide nanoparticles; medical imaging; stimuli-responsive; theranostics.
© 2020 Wiley-VCH GmbH.
Figures






Similar articles
-
Advances in Atherosclerosis Theranostics Harnessing Iron Oxide-Based Nanoparticles.Adv Sci (Weinh). 2024 May;11(17):e2308298. doi: 10.1002/advs.202308298. Epub 2024 Feb 17. Adv Sci (Weinh). 2024. PMID: 38368274 Free PMC article. Review.
-
Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications.Adv Drug Deliv Rev. 2019 Jan 1;138:302-325. doi: 10.1016/j.addr.2019.01.005. Epub 2019 Jan 11. Adv Drug Deliv Rev. 2019. PMID: 30639256 Free PMC article. Review.
-
Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics.Int J Pharm. 2015 Dec 30;496(2):191-218. doi: 10.1016/j.ijpharm.2015.10.058. Epub 2015 Oct 28. Int J Pharm. 2015. PMID: 26520409 Review.
-
Magnetic nanoparticles for precision oncology: theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy.Nanomedicine (Lond). 2017 Jan;12(1):73-87. doi: 10.2217/nnm-2016-0316. Epub 2016 Nov 23. Nanomedicine (Lond). 2017. PMID: 27876448 Free PMC article. Review.
-
Chemical synthesis and assembly of uniformly sized iron oxide nanoparticles for medical applications.Acc Chem Res. 2015 May 19;48(5):1276-85. doi: 10.1021/acs.accounts.5b00038. Epub 2015 Apr 29. Acc Chem Res. 2015. PMID: 25922976 Review.
Cited by
-
New Types of Magnetic Nanoparticles for Stimuli-Responsive Theranostic Nanoplatforms.Adv Sci (Weinh). 2024 Feb;11(8):e2305459. doi: 10.1002/advs.202305459. Epub 2023 Nov 21. Adv Sci (Weinh). 2024. PMID: 37988692 Free PMC article. Review.
-
Nanoparticles in Clinical Translation for Cancer Therapy.Int J Mol Sci. 2022 Feb 1;23(3):1685. doi: 10.3390/ijms23031685. Int J Mol Sci. 2022. PMID: 35163607 Free PMC article. Review.
-
Protease-Mediated T1 Contrast Enhancement of Multilayered Magneto-Gadolinium Nanostructures for Imaging and Magnetic Hyperthermia.ACS Appl Mater Interfaces. 2024 Feb 14;16(6):6743-6755. doi: 10.1021/acsami.3c13914. Epub 2024 Jan 31. ACS Appl Mater Interfaces. 2024. PMID: 38295315 Free PMC article.
-
Defect-engineered magnetic bismuth nanomedicine for dual-modal imaging and synergistic lung tumor therapy.Mater Today Bio. 2025 Mar 18;32:101680. doi: 10.1016/j.mtbio.2025.101680. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40225134 Free PMC article.
-
Recent trends in preparation and biomedical applications of iron oxide nanoparticles.J Nanobiotechnology. 2024 Jan 8;22(1):24. doi: 10.1186/s12951-023-02235-0. J Nanobiotechnology. 2024. PMID: 38191388 Free PMC article. Review.
References
-
- Angelova M, Mlecnik B, Vasaturo A, Bindea G, Fredriksen T, Lafontaine L, Buttard B, Morgand E, Bruni D, Jouret-Mourin A, Hubert C, Kartheuser A, Humblet Y, Ceccarelli M, Syed N, Marincola FM, Bedognetti D, Van den Eynde M, Galon J, Cell 2018, 175, 751; - PubMed
- Hanahan D, Weinberg RA, Cell 2011, 144, 646; - PubMed
- Hu BC, Nature 2019, 574, 187; - PMC - PubMed
- Rozenblatt-Rosen O, Regev A, Oberdoerffer P, Nawy T, Hupalowska A, Rood JE, Ashenberg O, Cerami E, Coffey RJ, Demir E, Ding L, Esplin ED, Ford JM, Goecks J, Ghosh S, Gray JW, Guinney J, Hanlon SE, Hughes SK, Hwang ES, Iacobuzio-Donahue CA, Jane-Valbuena J, Johnson BE, Lau KS, Lively T, Mazzilli SA, Pe’er D, Santagata S, Shalek AK, Schapiro D, Snyder MP, Sorger PK, Spira AE, Srivastava S, Tan K, West RB, Williams EH, Human Tumor Atlas N, Cell 2020, 181, 236; - PubMed
- Elliot A, Myllymaki H, Feng Y, Cells 2020, 9; - PMC - PubMed
- Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM, Hellmann MD, Barron DA, Schram AM, Hameed M, Dogan S, Ross DS, Hechtman JF, DeLair DF, Yao J, Mandelker DL, Cheng DT, Chandramohan R, Mohanty AS, Ptashkin RN, Jayakumaran G, Prasad M, Syed MH, Rema AB, Liu ZY, Nafa K, Borsu L, Sadowska J, Casanova J, Bacares R, Kiecka IJ, Razumova A, Son JB, Stewart L, Baldi T, Mullaney KA, Al-Ahmadie H, Vakiani E, Abeshouse AA, Penson AV, Jonsson P, Camacho N, Chang MT, Won HH, Gross BE, Kundra R, Heins ZJ, Chen HW, Phillips S, Zhang H, Wang J, Ochoa A, Wills J, Eubank M, Thomas SB, Gardos SM, Reales DN, Galle J, Durany R, Cambria R, Abida W, Cercek A, Feldman DR, Gounder MM, Hakimi AA, Harding JJ, Iyer G, Janjigian YY, Jordan EJ, Kelly CM, Lowery MA, Morris LGT, Omuro AM, Raj N, Razavi P, Shoushtari AN, Shukla N, Soumerai TE, Varghese AM, Yaeger R, Coleman J, Bochner B, Riely GJ, Saltz LB, Scher HI, Sabbatini PJ, Robson ME, Klimstra DS, Taylor BS, Baselga J, Schultz N, Hyman DM, Arcila ME, Solit DB, Ladanyi M, Berger MF, Nat Med 2017, 23, 703; - PMC - PubMed
- Beltran H, Eng K, Mosquera JM, Sigaras A, Romanel A, Rennert H, Kossai M, Pauli C, Faltas B, Fontugne J, Park K, Banfelder J, Prandi D, Madhukar N, Zhang T, Padilla J, Greco N, McNary TJ, Herrscher E, Wilkes D, MacDonald TY, Xue H, Vacic V, Emde AK, Oschwald D, Tan AY, Chen Z, Collins C, Gleave ME, Wang Y, Chakravarty D, Schiffman M, Kim R, Campagne F, Robinson BD, Nanus DM, Tagawa ST, Xiang JZ, Smogorzewska A, Demichelis F, Rickman DS, Sboner A, Elemento O, Rubin MA, JAMA Oncol 2015, 1, 466. - PMC - PubMed
-
- Bedognetti D, Ceccarelli M, Galluzzi L, Lu R, Palucka K, Samayoa J, Spranger S, Warren S, Wong KK, Ziv E, Chowell D, Coussens LM, De Carvalho DD, DeNardo DG, Galon J, Kaufman HL, Kirchhoff T, Lotze MT, Luke JJ, Minn AJ, Politi K, Shultz LD, Simon R, Thorsson V, Weidhaas JB, Ascierto ML, Ascierto PA, Barnes JM, Barsan V, Bommareddy PK, Bot A, Church SE, Ciliberto G, De Maria A, Draganov D, Ho WS, McGee HM, Monette A, Murphy JF, Nistico P, Park W, Patel M, Quigley M, Radvanyi L, Raftopoulos H, Rudqvist NP, Snyder A, Sweis RF, Valpione S, Zappasodi R, Butterfield LH, Disis ML, Fox BA, Cesano A, Marincola FM, Society F for Immunotherapy of Cancer Cancer Immune Responsiveness Task, G. Working, J Immunother Cancer 2019, 7, 131; - PMC - PubMed
- Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF, Nat Med 2018, 24, 541. - PMC - PubMed
-
- Ruffell B, Coussens LM, Cancer Cell 2015, 27, 462; - PMC - PubMed
- Lewis CE, Harney AS, Pollard JW, Cancer Cell 2016, 30, 18; - PMC - PubMed
- Jansen RP, Handelsman DJ, Boylan LM, Conway A, Shearman RP, Fraser IS, Anderson JC, Fertil Steril 1987, 48, 39; - PubMed
- Yasuma T, Saito S, Kihara K, Acta Pathol Jpn 1988, 38, 723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

