Report of a rare case of congenital mitral valve prolapse with chronic kidney disease--reconsidered genotype-phenotypic correlations
- PMID: 33225636
- PMCID: PMC7963429
- DOI: 10.1002/mgg3.1558
Report of a rare case of congenital mitral valve prolapse with chronic kidney disease--reconsidered genotype-phenotypic correlations
Abstract
Background: Mitral valve prolapse (MVP) is a common cardiovascular disease defined as a late systolic click or mitral valve lobes that move up into the left atrium during ventricular systole, with or without mitral insufficiency. Dachsous catherin-related 1 (DCHS1) is one of the two known pathogenic genes associated with MVP. However, there is little information about the renal dysfunction caused by MVP and DCHS1 mutations.
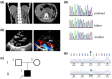
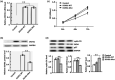
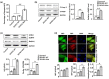
Methods: We analyzed the genetic etiology in a rare case of 9-year-old boy affected by chronic renal failure with MVP. Subsequently, we constructed stable cell lines overexpressing wild-type DCHS1 or mutant DCHS1 (c.8309G>A, p.R2770Q) to evaluate the influence of the DCHS1 mutation on the proliferation, apoptosis, and autophagy.
Results: Complete exome sequencing and pedigree verification revealed a mutation p.R2770Q (c.8309G>A) in exon 21 of the DCHS1 gene carried by the patient, which may affect the DNA binding. No such mutation was detected in his parents, indicating that this was a new mutation. Potential functional impact of sequence variants was predicted using in silico prediction programs including SIFT, Polyphen2, and Condel. This variant was determined to be a pathogenic mutation that has not been reported elsewhere. Subsequently, we used a stable DCHS1 gene-mutated HK-2 cell line to analyse proliferation, apoptosis, and autophagy, showed that kidney volume decreased with increasing cell death associated with a reduced proliferation.
Conclusions: Our analysis revealed a heterozygous variation of DCHS1 in a child with MVP. Our observations highlight previously unrecognized phenotypes of the currently recognized MVP genotype, including distinct chronic renal failure.
Keywords: DCHS1 mutation; chronic renal failure; genotype-phenotype correlations; mitral valve prolapse.
© 2020 The Authors. Molecular Genetics & Genomic Medicine published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mutations in DCHS1 cause mitral valve prolapse.Nature. 2015 Sep 3;525(7567):109-13. doi: 10.1038/nature14670. Epub 2015 Aug 10. Nature. 2015. PMID: 26258302 Free PMC article.
-
Deleterious variants in DCHS1 are prevalent in sporadic cases of mitral valve prolapse.Mol Genet Genomic Med. 2018 Jan;6(1):114-120. doi: 10.1002/mgg3.347. Epub 2017 Dec 10. Mol Genet Genomic Med. 2018. PMID: 29224215 Free PMC article.
-
Genetics of syndromic and non-syndromic mitral valve prolapse.Heart. 2018 Jun;104(12):978-984. doi: 10.1136/heartjnl-2017-312420. Epub 2018 Jan 19. Heart. 2018. PMID: 29352010 Free PMC article. Review.
-
Identification of known and unknown genes associated with mitral valve prolapse using an exome slice methodology.J Med Genet. 2020 Dec;57(12):843-850. doi: 10.1136/jmedgenet-2019-106715. Epub 2020 Apr 10. J Med Genet. 2020. PMID: 32277046
-
Genetic background of mitral valve prolapse.Rev Cardiovasc Med. 2022 Mar 12;23(3):96. doi: 10.31083/j.rcm2303096. Rev Cardiovasc Med. 2022. PMID: 35345263 Review.
Cited by
-
Metformin rescues migratory deficits of cells derived from patients with periventricular heterotopia.EMBO Mol Med. 2023 Oct 11;15(10):e16908. doi: 10.15252/emmm.202216908. Epub 2023 Aug 23. EMBO Mol Med. 2023. PMID: 37609821 Free PMC article.
References
-
- Basso, C. , Perazzolo Marra, M. , Rizzo, S. , De Lazzari, M. , Giorgi, B. , Cipriani, A. , Frigo, A. C. , Rigato, I. , Migliore, F. , Pilichou, K. , Bertaglia, E. , Cacciavillani, L. , Bauce, B. , Corrado, D. , Thiene, G. , & Iliceto, S. (2015). Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation, 132(7), 556–566. - PubMed
-
- Fulton, B. L. , Liang, J. J. , Enriquez, A. , Garcia, F. C. , Supple, G. E. , Riley, M. P. , Schaller, R. D. , Dixit, S. , Callans, D. J. , Marchlinski, F. E. , & Han, Y. (2018). Imaging characteristics of papillary muscle site of origin of ventricular arrhythmias in patients with mitral valve prolapse. Journal of Cardiovascular Electrophysiology, 29(1), 146–153. 10.1111/jce.13374 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

