Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells
- PMID: 33225894
- PMCID: PMC7681996
- DOI: 10.1186/s12866-020-02023-y
Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells
Abstract
Background: Bifidobacterium longum subsp. infantis (B. infantis) is a commensal bacterium that colonizes the gastrointestinal tract of breast-fed infants. B. infantis can efficiently utilize the abundant supply of oligosaccharides found in human milk (HMO) to help establish residence. We hypothesized that metabolites from B. infantis grown on HMO produce a beneficial effect on the host.
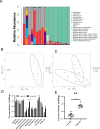
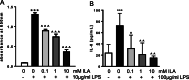
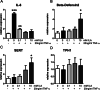
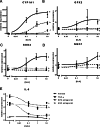
Results: In a previous study, we demonstrated that B. infantis routinely dominated the fecal microbiota of a breast fed Bangladeshi infant cohort (1). Characterization of the fecal metabolome of binned samples representing high and low B. infantis populations from this cohort revealed higher amounts of the tryptophan metabolite indole-3-lactic acid (ILA) in feces with high levels of B. infantis. Further in vitro analysis confirmed that B. infantis produced significantly greater quantities of the ILA when grown on HMO versus lactose, suggesting a growth substrate relationship to ILA production. The direct effects of ILA were assessed in a macrophage cell line and intestinal epithelial cell lines. ILA (1-10 mM) significantly attenuated lipopolysaccharide (LPS)-induced activation of NF-kB in macrophages. ILA significantly attenuated TNF-α- and LPS-induced increase in the pro-inflammatory cytokine IL-8 in intestinal epithelial cells. ILA increased mRNA expression of the aryl hydrogen receptor (AhR)-target gene CYP1A1 and nuclear factor erythroid 2-related factor 2 (Nrf2)-targeted genes glutathione reductase 2 (GPX2), superoxide dismutase 2 (SOD2), and NAD(P) H dehydrogenase (NQO1). Pretreatment with either the AhR antagonist or Nrf-2 antagonist inhibited the response of ILA on downstream effectors.
Conclusions: These findings suggest that ILA, a predominant metabolite from B. infantis grown on HMO and elevated in infant stool high in B. infantis, and protects gut epithelial cells in culture via activation of the AhR and Nrf2 pathway.
Keywords: Aryl-hydrocarbon receptor; Indole-3-lactic acid; Milk oligosaccharides; Nuclear factor erythroid 2–related factor 2.
Conflict of interest statement
DAM, DB and CBL are cofounders of Evolve Biosystems, a company focused on diet-based manipulation of the gut microbiota. Evolve Biosystems played no role in the origination, design, execution, interpretation, or publication of this work.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

