Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy)
- PMID: 33226133
- PMCID: PMC8094271
- DOI: 10.1002/14651858.CD008994.pub3
Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy)
Abstract
Background: Uterine fibroids can cause heavy menstrual bleeding. Medical treatments are considered to preserve fertility. It is unclear whether progestogens or progestogen-releasing intrauterine systems can reduce fibroid-related symptoms. This is the first update of a Cochrane Review published in 2013.
Objectives: To determine the effectiveness of progestogens or progestogen-releasing intrauterine systems in treating premenopausal women with uterine fibroids.
Search methods: We searched the Cochrane Gynaecology and Fertility Group Specialised Register, CENTRAL, MEDLINE, Embase, and PsycINFO databases to July 2020. We also searched trials registers for ongoing and registered trials, and checked references of relevant trials.
Selection criteria: All identified published or unpublished randomised controlled trials (RCTs) assessing the effect of progestogens or progestogen-releasing intrauterine systems in treating premenopausal women with uterine fibroids.
Data collection and analysis: Two authors independently extracted data, assessed risk of bias, and assessed the quality of the evidence using the GRADE approach.
Main results: This updated review included four studies with 221 women with uterine fibroids. The evidence was very low quality, downgraded for serious risk of bias, due to poor reporting of study methods, and serious imprecision. Levonorgestrel-releasing intrauterine device (LNG-IUS) versus hysterectomy There was no information on the outcomes of interest, including adverse events. LNG-IUS versus low dose combined oral contraceptive (COC) At 12 months, we are uncertain whether LNG-IUS reduced the percentage of abnormal uterine bleeding, measured with the alkaline hematin test (mean difference (MD) 77.50%, 95% confidence interval (CI) 70.44 to 84.56; 1 RCT, 44 women; very low-quality evidence), or the pictorial blood assessment chart (PBAC; MD 34.50%, 95% CI 11.59 to 57.41; 1 RCT, 44 women; very low-quality evidence); increased haemoglobin levels (MD 1.50 g/dL, 95% CI 0.85 to 2.15; 1 RCT, 44 women; very low-quality evidence), or reduced fibroid size more than COC (MD 1.90%, 95% CI -12.24 to 16.04; 1 RCT, 44 women; very low-quality evidence). The study did not measure adverse events. LNG-IUS versus oral progestogen (norethisterone acetate (NETA)) Compared to NETA, we are uncertain whether LNG-IUS reduced abnormal uterine bleeding more from baseline to six months (visual bleeding score; MD 23.75 points, 95% CI 1.26 to 46.24; 1 RCT, 45 women; very low-quality evidence); increased the percentage of change in haemoglobin from baseline to three months (MD 4.53%, 95% CI 1.46 to 7.60; 1 RCT, 48 women; very low-quality evidence), or from baseline to six months (MD 10.14%, 95% CI 5.57 to 14.71; 1 RCT, 45 women; very low-quality evidence). The study did not measure fibroid size. Spotting (adverse event) was more likely to be reported by women with the LNG-IUS (64.3%) than by those taking NETA (30%; 1 RCT, 45 women; very low-quality evidence). Oral progestogen (dienogest, desogestrel) versus goserelin acetate Compared to goserelin acetate, we are uncertain whether abnormal uterine bleeding was reduced at 12 weeks with dienogest (PBAC; MD 216.00 points, 95% CI 149.35 to 282.65; 1 RCT, 14 women; very low-quality evidence) or desogestrel (PBAC; MD 78.00 points, 95% CI 28.94 to 127.06; 1 RCT, 16 women; very low-quality evidence). Vasomotor symptoms (adverse events, e.g. hot flashes) are only associated with goserelin acetate (55%), not with dienogest (1 RCT, 14 women; very low-quality evidence) or with desogestrel (1 RCT, 16 women; very low-quality evidence). The study did not report fibroid size.
Authors' conclusions: Because of very low-quality evidence, we are uncertain whether the LNG-IUS reduces abnormal uterine bleeding or increases haemoglobin levels in premenopausal women with uterine fibroids, compared to COC or norethisterone acetate. There was insufficient evidence to determine whether the LNG-IUS reduces the size of uterine fibroids compared to COC. We are uncertain whether oral progestogens reduce abnormal uterine bleeding as effectively as goserelin acetate, but women reported fewer adverse events, such as hot flashes.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
None known
Figures
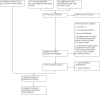

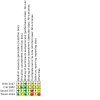

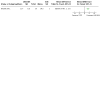





Update of
-
Progestogens or progestogen-releasing intrauterine systems for uterine fibroids.Cochrane Database Syst Rev. 2013 Feb 28;(2):CD008994. doi: 10.1002/14651858.CD008994.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2020 Nov 23;11:CD008994. doi: 10.1002/14651858.CD008994.pub3. PMID: 23450594 Updated.
References
References to studies included in this review
Brito 2017 {published data only}
-
- Brito LGO, Vieira CS, Rosa ESilva JC, Ferriani RA, Nogueira AA. Treatment of abnormal uterine bleeding secondary to uterine fibroids-a pilot, randomized study with dienogest, desogestrel and goserelin acetate. Journal of Minimally Invasive Gynecology 2017;24 Suppl 1(7):S182.
Inki 2002 {published data only}
-
- Inki P, Hurskainen R, Palo P, Ekholm E, Grenman S, Kivela A, et al. Comparison of ovarian cyst formation in women using the levonorgestrel-releasing intrauterine system vs. hysterectomy. Ultrasound in Obstetrics & Gynecology 2002;20(4):381-5. - PubMed
Sayed 2011 {published data only}
-
- Sayed GH, Zakherah MS, El-Nashar SA, Shaaban MM. A randomized clinical trial of a levonorgestrel-releasing intrauterine system and a low-dose combined oral contraceptive for fibroid-related menorrhagia. International Journal of Gynaecology and Obstetrics 2011;112(2):126-30. - PubMed
Tosun 2014 {published data only}
References to studies excluded from this review
Archer 2017 {published data only}
-
- Archer DF, Stewart EA, Jain RI, Feldman RA, Lukes AS, North JD, et al. Elagolix for the management of heavy menstrual bleeding associated with uterine fibroids: results from a phase 2a proof-of-concept study. Fertility and Sterility 2017;108(1):152-60.e4. - PubMed
Bigatti 2014 {published data only}
-
- Bigatti G, Iemmello R, Desgro M, Pollino S, Santirocco M. Progesteron preoperative treatment for myomectomy with the intrauterine bigatti shaver (IBS). Gynecological Surgery 2014;11:119-20.
Caird 1997 {published data only}
-
- Caird LE, West CP, Lumsden MA, Hannan WJ, Gow SM. Medroxyprogesterone acetate with zoladex for long-term treatment of fibroids: effects on bone density and patient acceptability. Human Reproduction 1997;12(3):436-40. - PubMed
Carr 1993 {published data only}
-
- Carr BR, Marshburn PB, Weatherall PT, Bradshaw KD, Breslau NA, Byrd W, et al. An evaluation of the effect of gonadotropin-releasing hormone analogs and medroxyprogesterone acetate on uterine leiomyomata volume by magnetic resonance imaging: a prospective, randomized, double blind, placebo-controlled, crossover trial. Journal of Clinical Endocrinology and Metabolism 1993;76(5):1217-23. - PubMed
Carr 2018 {published data only}
-
- Carr BR, Stewart EA, Archer DF, Al-Hendy A, Bradley L, Watts NB, et al. Elagolix alone or with add-back therapy in women with heavy menstrual bleeding and uterine leiomyomas: a randomized controlled trial. Obstetrics and Gynecology 2018;Nov;132(5):1252-64. [DOI: 10.1097/AOG.0000000000002933] - DOI - PMC - PubMed
Chan 2007 {published data only}
-
- Chan SS, Tam WH, Yeo W, Yu MM, Ng DP, Wong AW, et al. A randomised controlled trial of prophylactic levonorgestrel intrauterine system in tamoxifen-treated women. International Journal of Gynaecology and Obstetrics 2007;114(12):1510-5. - PubMed
Chwalisz 2005 {published data only}
-
- Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocrine Reviews 2005;26(3):423-38. - PubMed
Chwalisz 2007 {published data only}
-
- Chwalisz K, Larsen L, Mattia-Goldberg C, Edmonds A, Elger W, Winkel CA. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertility and Sterility 2007;87(6):1399-412. - PubMed
Erkayıran 2018 {published data only}
-
- *Erkayıran U, Köstü B, Özer A, Tok A, Karaküçük S. Levonorgestrel intrauterine device reduces uterine fibroid size and improves related symptoms. International Journal of Research (IJR) 2018;6:341-7.
-
- Özer A, Köstü B, Ercan Ö, Pektas MK, Sakalli H. Levonorgestrel intrauterine device reduces uterine fibroid size and improves related symptoms. In: Journal of The Turkish German Gynecological Association. Vol. 17. 2016:S226.
Friedman 1988 {published data only}
-
- Friedman AJ, Barbieri RL, Doubilet PM, Fine C, Schiff I. A randomized, double-blind trial of a gonadotropin releasing-hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertility and Sterility 1988;49(3):404-9. - PubMed
Koh 2007 {published data only}
-
- Koh SC, Singh K. The effect of levonorgestrel-releasing intrauterine system use on menstrual blood loss and the hemostatic, fibrinolytic/inhibitor systems in women with menorrhagia. Journal of Thrombosis and Haemostasis 2007;5(1):133-8. - PubMed
Kriplani 2012 {published data only}
-
- Kriplani A, Awasthi D, Kulshrestha V, Agarwal N. Efficacy of the levonorgestrel-releasing intrauterine system in uterine leiomyoma. International Journal of Gynaecology and Obstetrics 2012;116(1):35-8. - PubMed
Kulshrestha 2012 {published data only}
-
- Kulshrestha V, Kriplani A, Agarwal N, Kachhawa G, Bhatla N. Comparison of efficacy of levonorgestrel intrauterine system (LNG‐IUS) in abnormal uterine bleeding due to dysfunctional uterine bleeding, uterine‐leiomyoma and adenomyosis. In: International Journal of Gynaecology and Obstetrics. Vol. 119. 2012:S395.
Lagana 2016 {published data only}
-
- Laganà AS, Giacobbe V, Triolo O, Granese R, Ban Frangež H, Vrtačnik-Bokal E, et al. Dienogest as preoperative treatment of submucous myomas for hysteroscopic surgery: a prospective, randomized study. Gynecological Endocrinology 2016;32(5):408-11. - PubMed
Lang 2018 {published data only}
-
- Lang J, Yu Q, Zhang S, Li H, Gude K, Ludwig C, et al. Dienogest for treatment of endometriosis in Chinese women: a placebo-controlled, randomized, double-blind phase 3 study. Journal of Women's Health 2018;27(2):148-55. - PubMed
Levens 2008 {published data only}
Magalhaes 2010 {published data only}
-
- Magalhaes J, Magalhaes LB. Uterine volume and menstrual patterns in users of LNG-IUS intrauterine system with idiopathic menorrhagia or menorrhagia due to leiomyomas. A cohort prospective study of 8 years. In: European Journal of Contraception and Reproductive Health Care. Vol. 15. 2010:FC-34.
Palomba 2002 {published data only}
-
- Palomba S, Sena T, Morelli M, Noia R, Zullo F, Mastrantonio P. Effect of different doses of progestin on uterine leiomyomas in postmenopausal women. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2002;102(2):199-201. - PubMed
Rodriguez 2010 {published and unpublished data}
-
- Rodriguez MI, Warden M, Darney PD. Intrauterine progestins, progesterone antagonists, and receptor modulators: a review of gynecologic applications. American Journal of Obstetrics and Gynecology 2010;202(5):420-8. - PubMed
Scialli 1995 {published data only}
-
- Scialli AR, Jestila KJ. Sustained benefits of leuprolide acetate with or without subsequent medroxyprogesterone acetate in the nonsurgical management of leiomyomata uteri. Fertility and Sterility 1995;64(2):313-20. - PubMed
Siddiqui 2008 {published and unpublished data}
-
- Siddiqui M, Hossain F, Banu L. Levonorgestrel intrauterine system Mirena: an update. Bangladesh Journal of Obstetrics and Gynecology 2008;23(1):25-31.
Soysal 2005 {published data only}
-
- Soysal S, Soysal ME. The efficacy of levonorgestrel-releasing intrauterine device in selected cases of myoma-related menorrhagia: a prospective controlled trial. Gynecologic and Obstetric Investigation 2005;59(1):29-35. - PubMed
Verspyck 2000 {published data only}
-
- Verspyck E, Marpeau L, Lucas C. Leuprorelin depot 3.75 mg versus lynestrenol in the preoperative treatment of symptomatic uterine myomas: a multicentre randomised trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2000;89:7-13. - PubMed
West 1992 {published data only}
-
- West CP, Lumsden MA, Hillier H, Sweeting V, Baird DT. Potential role for medroxyprogesterone acetate as an adjunct to goserelin (Zoladex) in the medical management of uterine fibroids. Human Reproduction 1992;7(3):328-32. - PubMed
Wilkens 2008 {published data only}
-
- Wilkens J, Chwalisz K, Han C, Walker J, Cameron IT, Ingamells S, et al. Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity, and clinical symptoms in patients with uterine leiomyomata scheduled for hysterectomy. Journal of Clinical Endocrinology and Metabolism 2008;93(12):4664-71. - PubMed
Yoshida 2010 {published and unpublished data}
-
- Yoshida S, Ohara N, Xu Q, Chen W, Wang J, Nakabayashi K, et al. Cell-type specific actions of progesterone receptor modulators in the regulation of uterine leiomyoma growth. Seminars in Reproductive Medicine 2010;28(3):260-73. - PubMed
References to studies awaiting assessment
NCT01738724 {published data only}
-
- NCT01738724. Study of the efficacy of dienogest in the treatment of uterine leiomyomas when compared to desogestrel and goserelin. clinicaltrials.gov/show/NCT01738724 (first posted 30 November 2012).
References to ongoing studies
2014‐003408‐65 {published data only}
-
- 2014-003408-65. Ulipristal acetate versus conventional management of heavy menstrual bleeding (HMB; including uterine fibroids): a randomised controlled trial and exploration of mechanism of action. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:201... (first posted 2 December 2014).
Additional references
Andersen 1993
-
- Andersen J, Grine E, Eng CL, Zhao K, Barbieri RL, Chumas JC, et al. Expression of connexin-43 in human myometrium and leiomyoma. American Journal of Obstetrics and Gynecology 1993;169:1266-76. - PubMed
Covidence [Computer program]
-
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed January 2019. Available at covidence.org.
David 2005
-
- David M, Ebert AD. Treatment of uterine fibroids by embolization – advantages, disadvantages, and pitfalls. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2005;123:131–8. - PubMed
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG, editor(s), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
FIGO 2011
-
- Munro MG, Critchley HO, Broder MS, Fraser IS, FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. International Journal of Gynaecology and Obstetrics 2011;113(1):3-13. - PubMed
Flake 2003
Glanc 2008
-
- Glanc P, Betel C, LevToaff A. Sonohysterography: technique and clinical applications. Ultrasound Clinics 2008;3:427–31.
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 4 December 2018. Available at gradepro.org.
Gupta 2008
-
- Gupta S, Jose J, Manyonda I. Clinical presentation of fibroids. Best Practice & Research Clinical Obstetrics & Gynaecology 2008;22:615–26. - PubMed
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JC, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbooks.
Higgins 2011a
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbooks.
Higham 1990
-
- Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. BR J Obstet Gynaecol 1990;97:734-739. - PubMed
Jiang 2014
-
- Jiang W, Shen Q, Chen M, Wang Y, Zhou Q, Zhu X, et al. Levonorgestrel-releasing intrauterine system use in premenopausal women with symptomatic uterine leiomyoma: a systematic review. Steroids 2014;86:69-78. - PubMed
Kaunitz 2007
-
- Kaunitz AM. Progestin-releasing intrauterine systems and leiomyoma. Contraception 9 March 2007 [Epub ahead of print];75(6 Suppl):S130-3. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011) The Cochrane Collaboration. 2011. Available from training.cochrane.org/handbooks.
Lethaby 2015
Lethaby 2017
Lumbiganon 1996
-
- Lumbiganon P, Rugpao S, Phandhu-fung S, Laopaiboon M, Vudhikamraksa N, Werawatakul Y. Protective effect of depot-medroxyprogesterone acetate on surgically treated uterine leiomyomas: a multicentre case-control study. British Journal of Obstetrics and Gynaecology 1996;103(9):909-14. - PubMed
Magalhaes 2007
-
- Magalhaes J, Aldrighi JM, Lima GR. Uterine volume and menstrual patterns in users of the levonorgestrel-releasing intrauterine system with idiopathic menorrhagia or menorrhagia due to leiomyomas. Contraception 2007;75:193–8. - PubMed
Manzo 2000
-
- Maruo T, Matsuo H, Samoto T, Shimomura Y, Kurachi O, Gao Z, et al. Effects of progesterone on uterine leiomyoma growth and apoptosis. Steroids 2000;65(10-11):585-92. - PubMed
Marsh 2006
-
- Marsh EE, Bulun SE. Steroid hormones and leiomyomas. Obstetrics and Gynecology Clinics of North America 2006;33:59–67. - PubMed
Maruo 2004
-
- Maruo T, Ohara N, Wang J, Matsuo H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Human Reproduction Update 2004;10(3):207-20. - PubMed
Maruo 2007
-
- Maruo T, Ohara N, Matsuo H, Xu Q, Chen W, Sitruk-Ware R, et al. Effects of levonorgestrel-releasing IUS and progesterone receptor modulator PRM CDB-2914 on uterine leiomyomas. Contraception 21 March 2007 [Epub ahead of print];75(6 Suppl):S99-103. - PubMed
NICE 2018
-
- National Institute for Health and Clinical Excellence (NICE). Heavy menstrual bleeding: assessment and management (NG 88). www.nice.org.uk/guidance/ng88 (accessed 2 July 2020).
Rein 1995
-
- Rein MS, Barbieri RL, Friedman AJ. Progesterone: a critical role in the pathogenesis of uterine myomas. American Journal of Obstetrics and Gynecology 1995;172(1 Pt 1):14-8. - PubMed
Review Manager 2014 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ryan 2005
-
- Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clinical Obstetrics and Gynecology 2005;48:312–24. - PubMed
Silverberg 1986
-
- Silverberg SG, Haukkamaa M, Arko H, Nilsson CG, Luukkainen T. Endometrial morphology during long-term use of levonorgestrel-releasing intrauterine devices. International Journal of Gynecological Pathology 1986;5:235–41. - PubMed
Sterne 2017
-
- Sterne JA, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbooks.
Stewart 2013
Stewart 2017
-
- Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG 2017;124(10):1501-12. - PubMed
Stovall 2001
-
- Stovall DW. Clinical symptomatology of uterine leiomyomas. Clinical Obstetrics and Gynecology 2001;44:364–71. - PubMed
Sweeting 2004
Tang 2018
UN 2020
-
- United Nations, Department of Economic and Social Affairs, Population Division. World Contraceptive Use 2020 (POP/DB/CP/Rev2020). Available at www.un.org/en/development/desa/population/publications/dataset/contracep... 2020.
WHO 2015
-
- World Health Organization (WHO). Medical eligibility criteria for contraceptive use. Available at www.who.int/reproductivehealth/publications/family_planning/MEC-5/en/ 2015. - PubMed
WHO 2018
-
- World Health Organization (WHO). Family planning: a global handbook for providers (2018 update). Available at www.who.int/reproductivehealth/publications/fp-global-handbook/en/ 2018.
Zapata 2010
-
- Zapata LB, Whiteman MK, Tepper NK, Jamieson DJ, Marchbanks PA, Curtis KM. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception 2010;82(1):41-55. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

