Bile Acid Profiles in Primary Sclerosing Cholangitis and Their Ability to Predict Hepatic Decompensation
- PMID: 33226645
- PMCID: PMC8141059
- DOI: 10.1002/hep.31652
Bile Acid Profiles in Primary Sclerosing Cholangitis and Their Ability to Predict Hepatic Decompensation
Abstract
Background and aims: Altered bile acid (BA) homeostasis is an intrinsic facet of cholestatic liver diseases, but clinical usefulness of plasma BA assessment in primary sclerosing cholangitis (PSC) remains understudied. We performed BA profiling in a large retrospective cohort of patients with PSC and matched healthy controls, hypothesizing that plasma BA profiles vary among patients and have clinical utility.
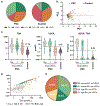
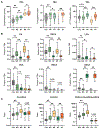
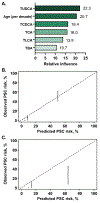
Approach and results: Plasma BA profiling was performed in the Clinical Biochemical Genetics Laboratory at Mayo Clinic using a mass spectrometry based assay. Cox proportional hazard (univariate) and gradient boosting machines (multivariable) models were used to evaluate whether BA variables predict 5-year risk of hepatic decompensation (HD; defined as ascites, variceal hemorrhage, or encephalopathy). There were 400 patients with PSC and 302 controls in the derivation cohort (Mayo Clinic) and 108 patients with PSC in the validation cohort (Norwegian PSC Research Center). Patients with PSC had increased BA levels, conjugated fraction, and primary-to-secondary BA ratios relative to controls. Ursodeoxycholic acid (UDCA) increased total plasma BA level while lowering cholic acid and chenodeoxycholic acid concentrations. Patients without inflammatory bowel disease (IBD) had primary-to-secondary BA ratios between those of controls and patients with ulcerative colitis. HD risk was associated with increased concentration and conjugated fraction of many BA, whereas higher G:T conjugation ratios were protective. The machine-learning model, PSC-BA profile score (concordance statistic [C-statistic], 0.95), predicted HD better than individual measures, including alkaline phosphatase, and performed well in validation (C-statistic, 0.86).
Conclusions: Patients with PSC demonstrated alterations of plasma BA consistent with known mechanisms of cholestasis, UDCA treatment, and IBD. Notably, BA profiles predicted future HD, establishing the clinical potential of BA profiling, which may be suited for use in clinical trials.
© 2020 by the American Association for the Study of Liver Diseases.
Figures



References
-
- Lazaridis KN, LaRusso NF. Primary Sclerosing Cholangitis. N Engl J Med 2016;375:2501–2502. - PubMed
-
- Fickert P, Wagner M. Biliary bile acids in hepatobiliary injury - What is the link? J Hepatol 2017;67:619–631. - PubMed
-
- de Krijger M, Wildenberg ME, de Jonge WJ, Ponsioen CY. Return to sender: Lymphocyte trafficking mechanisms as contributors to primary sclerosing cholangitis. J Hepatol 2019;71:603–615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

