Safety and Performance of the Tandem t:slim X2 with Control-IQ Automated Insulin Delivery System in Toddlers and Preschoolers
- PMID: 33226837
- PMCID: PMC8080923
- DOI: 10.1089/dia.2020.0507
Safety and Performance of the Tandem t:slim X2 with Control-IQ Automated Insulin Delivery System in Toddlers and Preschoolers
Abstract
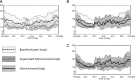
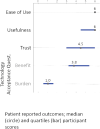
Background: Glycemic control is particularly challenging for toddlers and preschoolers with type 1 diabetes (T1D), and data on the use of closed-loop systems in this age range are limited. Materials and Methods: We studied use of a modified investigational version of the Tandem t:slim X2 Control-IQ system in children aged 2 to 5 years during 48 h in an outpatient supervised hotel (SH) setting followed by 3 days of home use to examine the safety of this system in young children. Meals and snacks were not restricted and boluses were estimated per parents' usual routine. At least 30 min of daily exercise was required during the SH phase. All participants were remotely monitored by study staff while on closed-loop in addition to monitoring by at least one parent throughout the study. Results: Twelve participants diagnosed with T1D for at least 3 months with mean age 4.7 ± 1.0 years (range 2.0-5.8 years) and hemoglobin A1c of 7.3% ± 0.8% were enrolled at three sites. With use of Control-IQ, the percentage of participants meeting our prespecified goals of less than 6% time below 70 mg/dL and less than 40% time above 180 mg/dL increased from 33% to 83%. Control-IQ use significantly improved percent time in range (70-180 mg/dL) compared to baseline (71.3 ± 12.5 vs. 63.7 ± 15.1, P = 0.016). All participants completed the study with no adverse events. Conclusions: In this brief pilot study, use of the modified Control-IQ system was safe in 2-5-year-old children with T1D and improved glycemic control.
Keywords: Artificial pancreas; Closed loop; Pediatrics; Type 1 diabetes.
Conflict of interest statement
L.E. has received consulting fees from Tandem. M.J.S. has received research funding from Insulet Corp., Medtronic, and Tandem. G.P.F. conducts research sponsored by Medtronic, Dexcom, Abbott, Tandem, Insulet, Beta Bionics, and Eli Lilly and has been a speaker/consultant/ad board member for Medtronic, Dexcom, Abbott, Tandem, Insulet, Beta Bionics, and Eli Lilly. M.D.D. has received research support from Tandem, Dexcom, and Medtronic. L.N. has no disclosures. L.H. owns stock in Tandem. R.K., E.B., C.B., and E.E. have no disclosures. B.A.B. has received research funding from Insulet Corp., Medtronic, Tandem, Dexcom, JDRF, and NIH and is on Medtronic, Novo Nordisk, and Convatec advisory boards. M.D.B. reports honorarium from Dexcom and Tandem; consulting revenue from Dexcom, Air Liquide, Healsy, and Adocia; research support from Dexcom, Novo Nordisk, Tandem, and Sanofi. RPW has received research funding from Tandem, Dexcom, Eli Lilly, and MannKind and received honorarium from Tandem and Eli Lilly.
Figures
References
-
- American Diabetes Association. 13. Children and adolescents: standards of medical care in diabetes-2020. Diabetes Care 2020;43(Suppl 1):S163–S182 - PubMed
-
- DiMeglio LA, Acerini CL, Codner E, et al. : ISPAD Clinical Practice Consensus Guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes 2018;19 Suppl 27:105–114 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials