Development and External Validation of a Machine Learning Tool to Rule Out COVID-19 Among Adults in the Emergency Department Using Routine Blood Tests: A Large, Multicenter, Real-World Study
- PMID: 33226957
- PMCID: PMC7713695
- DOI: 10.2196/24048
Development and External Validation of a Machine Learning Tool to Rule Out COVID-19 Among Adults in the Emergency Department Using Routine Blood Tests: A Large, Multicenter, Real-World Study
Abstract
Background: Conventional diagnosis of COVID-19 with reverse transcription polymerase chain reaction (RT-PCR) testing (hereafter, PCR) is associated with prolonged time to diagnosis and significant costs to run the test. The SARS-CoV-2 virus might lead to characteristic patterns in the results of widely available, routine blood tests that could be identified with machine learning methodologies. Machine learning modalities integrating findings from these common laboratory test results might accelerate ruling out COVID-19 in emergency department patients.
Objective: We sought to develop (ie, train and internally validate with cross-validation techniques) and externally validate a machine learning model to rule out COVID 19 using only routine blood tests among adults in emergency departments.
Methods: Using clinical data from emergency departments (EDs) from 66 US hospitals before the pandemic (before the end of December 2019) or during the pandemic (March-July 2020), we included patients aged ≥20 years in the study time frame. We excluded those with missing laboratory results. Model training used 2183 PCR-confirmed cases from 43 hospitals during the pandemic; negative controls were 10,000 prepandemic patients from the same hospitals. External validation used 23 hospitals with 1020 PCR-confirmed cases and 171,734 prepandemic negative controls. The main outcome was COVID 19 status predicted using same-day routine laboratory results. Model performance was assessed with area under the receiver operating characteristic (AUROC) curve as well as sensitivity, specificity, and negative predictive value (NPV).
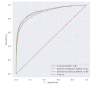
Results: Of 192,779 patients included in the training, external validation, and sensitivity data sets (median age decile 50 [IQR 30-60] years, 40.5% male [78,249/192,779]), AUROC for training and external validation was 0.91 (95% CI 0.90-0.92). Using a risk score cutoff of 1.0 (out of 100) in the external validation data set, the model achieved sensitivity of 95.9% and specificity of 41.7%; with a cutoff of 2.0, sensitivity was 92.6% and specificity was 59.9%. At the cutoff of 2.0, the NPVs at a prevalence of 1%, 10%, and 20% were 99.9%, 98.6%, and 97%, respectively.
Conclusions: A machine learning model developed with multicenter clinical data integrating commonly collected ED laboratory data demonstrated high rule-out accuracy for COVID-19 status, and might inform selective use of PCR-based testing.
Keywords: COVID-19; SARS-CoV-2; artificial intelligence; development; electronic medical records; emergency department; laboratory results; machine learning; model; testing; validation.
©Timothy B Plante, Aaron M Blau, Adrian N Berg, Aaron S Weinberg, Ik C Jun, Victor F Tapson, Tanya S Kanigan, Artur B Adib. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 02.12.2020.
Conflict of interest statement
Conflicts of Interest: TBP, AB, ASW, and ICJ have no disclosures. VFT received research funding from the National Institutes of Health (paid to the institution) for COVID-19–related clinical trials. ANB is a paid intern at Biocogniv. TSK and ABA have ownership of Biocogniv.
Figures
Similar articles
-
Rapid triage for COVID-19 using routine clinical data for patients attending hospital: development and prospective validation of an artificial intelligence screening test.Lancet Digit Health. 2021 Feb;3(2):e78-e87. doi: 10.1016/S2589-7500(20)30274-0. Epub 2020 Dec 11. Lancet Digit Health. 2021. PMID: 33509388 Free PMC article.
-
Routine Laboratory Blood Tests Predict SARS-CoV-2 Infection Using Machine Learning.Clin Chem. 2020 Nov 1;66(11):1396-1404. doi: 10.1093/clinchem/hvaa200. Clin Chem. 2020. PMID: 32821907 Free PMC article.
-
Rapid identification of SARS-CoV-2-infected patients at the emergency department using routine testing.Clin Chem Lab Med. 2020 Jun 29;58(9):1587-1593. doi: 10.1515/cclm-2020-0593. Print 2020 Aug 27. Clin Chem Lab Med. 2020. PMID: 32598302
-
Diagnosing COVID-19 in the Emergency Department: A Scoping Review of Clinical Examinations, Laboratory Tests, Imaging Accuracy, and Biases.Acad Emerg Med. 2020 Aug;27(8):653-670. doi: 10.1111/acem.14048. Epub 2020 Jul 26. Acad Emerg Med. 2020. PMID: 32542934 Free PMC article.
-
Real-time RT-PCR for COVID-19 diagnosis: challenges and prospects.Pan Afr Med J. 2020 Jul 21;35(Suppl 2):121. doi: 10.11604/pamj.supp.2020.35.24258. eCollection 2020. Pan Afr Med J. 2020. PMID: 33282076 Free PMC article. Review.
Cited by
-
A systematic review of prediction models to diagnose COVID-19 in adults admitted to healthcare centers.Arch Public Health. 2021 Jun 18;79(1):105. doi: 10.1186/s13690-021-00630-3. Arch Public Health. 2021. PMID: 34144711 Free PMC article. Review.
-
COVID-19 diagnosis from routine blood tests using artificial intelligence techniques.Biomed Signal Process Control. 2022 Feb;72:103263. doi: 10.1016/j.bspc.2021.103263. Epub 2021 Nov 1. Biomed Signal Process Control. 2022. PMID: 34745318 Free PMC article.
-
The accuracy of machine learning approaches using non-image data for the prediction of COVID-19: A meta-analysis.Int J Med Inform. 2022 Aug;164:104791. doi: 10.1016/j.ijmedinf.2022.104791. Epub 2022 May 13. Int J Med Inform. 2022. PMID: 35594810 Free PMC article. Review.
-
A machine learning PROGRAM to identify COVID-19 and other diseases from hematology data.Future Sci OA. 2021 Jun 12;7(7):FSO733. doi: 10.2144/fsoa-2020-0207. eCollection 2021 Aug. Future Sci OA. 2021. PMID: 34254032 Free PMC article.
-
A novel explainable COVID-19 diagnosis method by integration of feature selection with random forest.Inform Med Unlocked. 2022;30:100941. doi: 10.1016/j.imu.2022.100941. Epub 2022 Apr 6. Inform Med Unlocked. 2022. PMID: 35399333 Free PMC article.
References
-
- Covid in the U.S.: Latest Map and Case Count. The New York Times. [2020-11-23]. https://www.nytimes.com/interactive/2020/us/coronavirus-us-cases.html.
-
- Karow J. Scientists seek solution to coronavirus testing bottleneck. Modern Healthcare. [2020-08-04]. https://www.modernhealthcare.com/patients/scientists-seek-solution-coron....
-
- Goldstein BA, Navar AM, Carter RE. Moving beyond regression techniques in cardiovascular risk prediction: applying machine learning to address analytic challenges. Eur Heart J. 2017 Jun 14;38(23):1805–1814. doi: 10.1093/eurheartj/ehw302. http://europepmc.org/abstract/MED/27436868 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

