Hypofractionated Postprostatectomy Radiation Therapy for Prostate Cancer to Reduce Toxicity and Improve Patient Convenience: A Phase 1/2 Trial
- PMID: 33227441
- PMCID: PMC7965239
- DOI: 10.1016/j.ijrobp.2020.11.009
Hypofractionated Postprostatectomy Radiation Therapy for Prostate Cancer to Reduce Toxicity and Improve Patient Convenience: A Phase 1/2 Trial
Abstract
Purpose: The phase 1 portion of this multicenter, phase 1/2 study of hypofractionated (HypoFx) prostate bed radiation therapy (RT) as salvage or adjuvant therapy aimed to identify the shortest dose-fractionation schedule with acceptable toxicity. The phase 2 portion aimed to assess the health-related quality of life (QoL) of using this HypoFx regimen.
Methods and materials: Eligibility included standard adjuvant or salvage prostate bed RT indications. Patients were assigned to receive 1 of 3 daily RT schedules: 56.6 Gy in 20 Fx, 50.4 Gy in 15 Fx, or 42.6 Gy in 10 Fx. Regional nodal irradiation and androgen deprivation therapy were not allowed. Participants were followed for 2 years after treatment with outcome measures based on prostate-specific antigen levels, toxicity assessments (Common Terminology Criteria for Adverse Events, v4.0), QoL measures (the Expanded Prostate Cancer Index Composite [EPIC] and EuroQol EQ-5D instruments), and out-of-pocket costs.
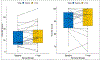
Results: There were 32 evaluable participants, and median follow-up was 3.53 years. The shortest dose-fractionation schedule with acceptable toxicity was determined to be 42.6 Gy in 10 Fx, with most patients (23) treated with this schedule. Grade 3 genitourinary (GU) and gastrointestinal (GI) toxicities occurred in 3 patients and 1 patient, respectively. There was 1 grade 4 sepsis event. Higher dose to the hottest 25% of the rectum was associated with increased risk of grade 2+ GI toxicity; no dosimetric factors were found to predict for GU toxicity. There was a significant decrease in the mean bowel, but not bladder, QoL score at 1 year compared with baseline. Prostate-specific antigen failure occurred in 34.3% of participants, using a definition of nadir plus 2 ng/mL. Metastases were more likely to occur in regional lymph nodes (5 of 7) than in bones (2 of 7). The mean out-of-pocket cost for patients during treatment was $223.90.
Conclusions: We identified 42.6 Gy in 10 fractions as the shortest dose-fractionation schedule with acceptable toxicity in this phase 1/2 study. There was a higher than expected rate of grade 2 to 3 GU and GI toxicity and a decreased EPIC bowel QoL domain with this regimen. Future studies are needed to explore alternative adjuvant/salvage HypoFx RT schedules after radical prostatectomy.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

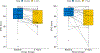

Comment in
-
In Regard to Wages et al and Leite et al.Int J Radiat Oncol Biol Phys. 2021 Aug 1;110(5):1548-1549. doi: 10.1016/j.ijrobp.2021.04.045. Int J Radiat Oncol Biol Phys. 2021. PMID: 34273332 No abstract available.
-
In Reply to Fiorino et al.Int J Radiat Oncol Biol Phys. 2021 Aug 1;110(5):1549-1550. doi: 10.1016/j.ijrobp.2021.04.046. Int J Radiat Oncol Biol Phys. 2021. PMID: 34273333 No abstract available.
References
-
- Swanson GP, Riggs M, Hermans M. Pathologic findings at radical prostatectomy: Risk factors for failure and death. Urol Oncol. 2007;25(2):110–114. - PubMed
-
- Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96–02/AUO AP 09/95. J Clin Oncol. 2009 June 20 2009;27(18):2924–2930. - PubMed
-
- Gandaglia G, Briganti A, Clarke N, et al. Adjuvant and salvage radiotherapy after radical prostatectomy in prostate cancer patients. Eur Urol. 2017;72:689–709. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

