Behavior and lineage progression of neural progenitors in the mammalian cortex
- PMID: 33227588
- PMCID: PMC8058148
- DOI: 10.1016/j.conb.2020.10.017
Behavior and lineage progression of neural progenitors in the mammalian cortex
Abstract
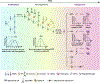
The cerebral cortex is a central structure in the mammalian brain that enables higher cognitive functions and intellectual skills. It is the hallmark of the mammalian nervous system with enormous complexity, consisting of a large number of neurons and glia that are diverse in morphology, molecular expression, biophysical properties, circuit connectivity and physiological function. Cortical neurons and glia are generated by neural progenitor cells during development. Ensuring the correct cell cycle kinetics, fate behavior and lineage progression of neural progenitor cells is essential to determine the number and types of neurons and glia in the cerebral cortex, which together constitute neural circuits for brain function. In this review, we discuss recent findings on mammalian cortical progenitor cell types and their lineage behaviors in generating neurons and glia, cortical evolution and expansion, and advances in brain organoid technology that allow the modeling of human cortical development under normal and disease conditions.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement
Nothing declared.
Figures


References
-
- Wonders CP, Anderson SA: The origin and specification of cortical interneurons. Nat Rev Neurosci 2006, 7:687–696. - PubMed
-
- Swinnen N, Smolders S, Avila A, Notelaers K, Paesen R, Ameloot M, Brone B, Legendre P, Rigo JM: Complex invasion pattern of the cerebral cortex bymicroglial cells during development of the mouse embryo. Glia 2013, 61:150–163. - PubMed
-
- Dzierzak E, Bigas A: Blood Development: Hematopoietic Stem Cell Dependence and Independence. Cell Stem Cell 2018, 22:639–651. - PubMed
-
- Florio M, Huttner WB: Neural progenitors, neurogenesis and the evolution of the neocortex. Development 2014, 141:2182–2194. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

