Suppression of Non-Random Fertilization by MHC Class I Antigens
- PMID: 33227981
- PMCID: PMC7699254
- DOI: 10.3390/ijms21228731
Suppression of Non-Random Fertilization by MHC Class I Antigens
Abstract
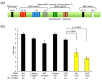
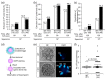
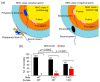
Hermaphroditic invertebrates and plants have a self-recognition system on the cell surface of sperm and eggs, which prevents their self-fusion and enhances non-self-fusion, thereby contributing to genetic variation. However, the system of sperm-egg recognition in mammals is under debate. To address this issue, we explored the role of major histocompatibility complex class I (MHC class I, also known as histocompatibility 2-Kb or H2-Kb and H2-Db in mice) antigens by analyzing H2-Kb-/-H2-Db-/-β2-microglobulin (β2M)-/- triple-knockout (T-KO) male mice with full fertility. T-KO sperm exhibited an increased sperm number in the perivitelline space of wild-type (WT) eggs in vitro. Moreover, T-KO sperm showed multiple fusion with zona pellucida (ZP)-free WT eggs, implying that the ability of polyspermy block for sperm from T-KO males was weakened in WT eggs. When T-KO male mice were intercrossed with WT female mice, the percentage of females in progeny increased. We speculate that WT eggs prefer fusion with T-KO sperm, more specifically X-chromosome-bearing sperm (X sperm), suggesting the presence of preferential (non-random) fertilization in mammals, including humans.
Keywords: MHC class I; non-random fertilization; polyspermy block; sex ratio.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

