Nutrients and Pathways that Regulate Health Span and Life Span
- PMID: 33228041
- PMCID: PMC7709628
- DOI: 10.3390/geriatrics5040095
Nutrients and Pathways that Regulate Health Span and Life Span
Abstract
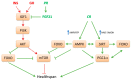
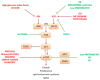
Both life span and health span are influenced by genetic, environmental and lifestyle factors. With the genetic influence on human life span estimated to be about 20-25%, epigenetic changes play an important role in modulating individual health status and aging. Thus, a main part of life expectance and healthy aging is determined by dietary habits and nutritional factors. Excessive or restricted food consumption have direct effects on health status. Moreover, some dietary interventions including a reduced intake of dietary calories without malnutrition, or a restriction of specific dietary component may promote health benefits and decrease the incidence of aging-related comorbidities, thus representing intriguing potential approaches to improve healthy aging. However, the relationship between nutrition, health and aging is still not fully understood as well as the mechanisms by which nutrients and nutritional status may affect health span and longevity in model organisms. The broad effect of different nutritional conditions on health span and longevity occurs through multiple mechanisms that involve evolutionary conserved nutrient-sensing pathways in tissues and organs. These pathways interacting each other include the evolutionary conserved key regulators mammalian target of rapamycin, AMP-activated protein kinase, insulin/insulin-like growth factor 1 pathway and sirtuins. In this review we provide a summary of the main molecular mechanisms by which different nutritional conditions, i.e., specific nutrient abundance or restriction, may affect health span and life span.
Keywords: aging; health span; life span; nutrient-sensing pathways; nutrients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

