Sonic Hedgehog Signature in Pediatric Primary Bone Tumors: Effects of the GLI Antagonist GANT61 on Ewing's Sarcoma Tumor Growth
- PMID: 33228057
- PMCID: PMC7699338
- DOI: 10.3390/cancers12113438
Sonic Hedgehog Signature in Pediatric Primary Bone Tumors: Effects of the GLI Antagonist GANT61 on Ewing's Sarcoma Tumor Growth
Abstract
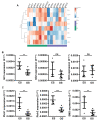
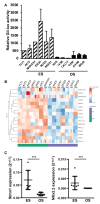
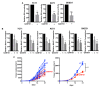
Osteosarcoma (OS) and Ewing's sarcoma (ES) are the most common malignant bone tumors in children and adolescents. In many cases, the prognosis remains very poor. The Sonic hedgehog (SHH) signaling pathway, strongly involved in the development of many cancers, regulate transcription via the transcriptional factors Gli1-3. In this context, RNAseq analysis of OS and ES cell lines reveals an increase of some major compounds of the SHH signaling cascade in ES cells, such as the transcriptional factor Gli1. This increase leads to an augmentation of the transcriptional response of Gli1 in ES cell lines, demonstrating a dysregulation of Gli1 signaling in ES cells and thus the rationale for targeting Gli1 in ES. The use of a preclinical model of ES demonstrates that GANT61, an inhibitor of the transcriptional factor Gli1, reduces ES primary tumor growth. In vitro experiments show that GANT61 decreases the viability of ES cell, mainly through its ability to induce caspase-3/7-dependent cell apoptosis. Taken together, these results demonstrates that GANT61 may be a promising therapeutic strategy for inhibiting the progression of primary ES tumors.
Keywords: GANT61; Gli1; SHH; ewing’s sarcoma; osteosarcoma; primary tumor growth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Grier H.E., Krailo M.D., Tarbell N.J., Link M.P., Fryer C.J.H., Pritchard D.J., Gebhardt M.C., Dickman P.S., Perlman E.J., Meyers P.A., et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N. Engl. J. Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. - DOI - PubMed
-
- Meyers P.A., Schwartz C.L., Krailo M.D., Healey J.H., Bernstein M.L., Betcher D., Ferguson W.S., Gebhardt M.C., Goorin A.M., Harris M., et al. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival—a report from the Children’s Oncology Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008;26:633–638. doi: 10.1200/JCO.2008.14.0095. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

