Wnt/β-Catenin Target Genes in Colon Cancer Metastasis: The Special Case of L1CAM
- PMID: 33228199
- PMCID: PMC7699470
- DOI: 10.3390/cancers12113444
Wnt/β-Catenin Target Genes in Colon Cancer Metastasis: The Special Case of L1CAM
Abstract
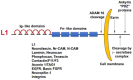
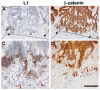
Cell adhesion to neighboring cells is a fundamental biological process in multicellular organisms that is required for tissue morphogenesis. A tight coordination between cell-cell adhesion, signaling, and gene expression is a characteristic feature of normal tissues. Changes, and often disruption of this coordination, are common during invasive and metastatic cancer development. The Wnt/β-catenin signaling pathway is an excellent model for studying the role of adhesion-mediated signaling in colorectal cancer (CRC) invasion and metastasis, because β-catenin has a dual role in the cell; it is a major adhesion linker of cadherin transmembrane receptors to the cytoskeleton and, in addition, it is also a key transducer of Wnt signaling to the nucleus, where it acts as a co-transcriptional activator of Wnt target genes. Hyperactivation of Wnt/β-catenin signaling is a common feature in the majority of CRC patients. We found that the neural cell adhesion receptor L1CAM (L1) is a target gene of β-catenin signaling and is induced in carcinoma cells of CRC patients, where it plays an important role in CRC metastasis. In this review, we will discuss studies on β-catenin target genes activated during CRC development (in particular, L1), the signaling pathways affected by L1, and the role of downstream target genes activated by L1 overexpression, especially those that are also part of the intestinal stem cell gene signature. As intestinal stem cells are highly regulated by Wnt signaling and are believed to also play major roles in CRC progression, unravelling the mechanisms underlying the regulation of these genes will shed light on both normal intestinal homeostasis and the development of invasive and metastatic CRC.
Keywords: EMT; L1; Lgr5; NF-κB; Wnt target genes; cancer stem cells; cell adhesion; colon cancer; invasion and metastasis; β-catenin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The Wnt Target Gene L1 in Colon Cancer Invasion and Metastasis.Cancers (Basel). 2016 May 11;8(5):48. doi: 10.3390/cancers8050048. Cancers (Basel). 2016. PMID: 27187476 Free PMC article. Review.
-
TRIB3 Interacts With β-Catenin and TCF4 to Increase Stem Cell Features of Colorectal Cancer Stem Cells and Tumorigenesis.Gastroenterology. 2019 Feb;156(3):708-721.e15. doi: 10.1053/j.gastro.2018.10.031. Epub 2018 Oct 24. Gastroenterology. 2019. PMID: 30365932
-
Induction of the intestinal stem cell signature gene SMOC-2 is required for L1-mediated colon cancer progression.Oncogene. 2016 Feb 4;35(5):549-57. doi: 10.1038/onc.2015.127. Epub 2015 Apr 27. Oncogene. 2016. PMID: 25915847
-
The intestinal stem cell regulating gene ASCL2 is required for L1-mediated colon cancer progression.Cancer Lett. 2018 Jun 28;424:9-18. doi: 10.1016/j.canlet.2018.03.022. Epub 2018 Mar 15. Cancer Lett. 2018. PMID: 29551399
-
Interaction of the Wnt/β-catenin and RAS-ERK pathways involving co-stabilization of both β-catenin and RAS plays important roles in the colorectal tumorigenesis.Adv Biol Regul. 2018 May;68:46-54. doi: 10.1016/j.jbior.2018.01.001. Epub 2018 Jan 10. Adv Biol Regul. 2018. PMID: 29449169 Review.
Cited by
-
RGL2 Drives the Metastatic Progression of Colorectal Cancer via Preventing the Protein Degradation of β-Catenin and KRAS.Cancers (Basel). 2021 Apr 7;13(8):1763. doi: 10.3390/cancers13081763. Cancers (Basel). 2021. PMID: 33917100 Free PMC article.
-
Integrated Analysis of CD1A Immune Infiltration and Competing Endogenous RNA Networks in COAD.Int J Gen Med. 2024 May 11;17:2037-2053. doi: 10.2147/IJGM.S455546. eCollection 2024. Int J Gen Med. 2024. PMID: 38751492 Free PMC article.
-
The Collagen-Modifying Enzyme PLOD2 Is Induced and Required during L1-Mediated Colon Cancer Progression.Int J Mol Sci. 2021 Mar 29;22(7):3552. doi: 10.3390/ijms22073552. Int J Mol Sci. 2021. PMID: 33805564 Free PMC article.
-
Reciprocal regulation of forkhead box C1 and L1 cell adhesion molecule contributes to triple-negative breast cancer progression.Breast Cancer Res Treat. 2024 Apr;204(3):465-474. doi: 10.1007/s10549-023-07177-7. Epub 2024 Jan 6. Breast Cancer Res Treat. 2024. PMID: 38183514 Free PMC article.
-
L1 cell adhesion molecule high expression is associated with poor prognosis in surgically resected brain metastases from lung adenocarcinoma.Clinics (Sao Paulo). 2022 May 4;77:100040. doi: 10.1016/j.clinsp.2022.100040. eCollection 2022. Clinics (Sao Paulo). 2022. PMID: 35525225 Free PMC article.
References
-
- Sharma R. Wingless, a new mutant in D. melanogaster. J. Dros. Inf. Service. 1973;50:134.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

