Emergence of Resistance to Integrase Strand Transfer Inhibitors during Dolutegravir Containing Triple-Therapy in a Treatment-Experienced Patient with Pre-Existing M184V/I Mutation
- PMID: 33228206
- PMCID: PMC7699495
- DOI: 10.3390/v12111330
Emergence of Resistance to Integrase Strand Transfer Inhibitors during Dolutegravir Containing Triple-Therapy in a Treatment-Experienced Patient with Pre-Existing M184V/I Mutation
Abstract
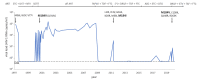
With the current widespread use of dolutegravir in low-income countries, the understanding of the impact of nucleoside reverse transcriptase inhibitor (NRTI-) associated mutations on the efficacy of dolutegravir-containing antiretroviral therapy (ART) is of utmost importance. We describe a rare case of a patient with pre-existing M184V/I mutation and virological failure on a dolutegravir/lamivudine/abacavir regimen with the emergence of integrase strand transfer inhibitor resistance mutations. Additional risk factors, which may have triggered the virological failure, included suboptimal adherence and low nadir CD4+ cell count. This case illustrates that dolutegravir-containing triple-therapy should be prescribed with caution to patients with pre-existing M184V/I mutation and poor efficacy of the reverse transcriptase inhibitor backbone. In addition, this case highlights the need for viral load monitoring in patients on dolutegravir-containing regimens in settings with a high prevalence of the M184V/I mutation such as in low-income countries.
Keywords: HIV-1; M184V/I mutation; antiretroviral therapy; dolutegravir; drug resistance; integrase strand transfer inhibitor; virological failure.
Conflict of interest statement
D.L.B. reports travel grants and honoraria from ViiV, MSD, and Gilead Sciences outside the submitted work. K.J.M. reports grants travel grants and honoraria from ViiV and Gilead Sciences outside the submitted work; the University of Zurich received an unrestricted research grant from Gilead Science for studies that Metzner serves as principal investigator, unrelated to the submitted work. H.F.G. has received unrestricted research grants from Gilead Sciences; fees for data and safety monitoring board or consulting/advisory board membership from Merck Gilead Sciences, ViiV, Sandoz, and Mepha.
Figures
Similar articles
-
Virological outcomes and genotypic resistance on dolutegravir-based antiretroviral therapy versus standard of care in children and adolescents: a secondary analysis of the ODYSSEY trial.Lancet HIV. 2025 Mar;12(3):e201-e213. doi: 10.1016/S2352-3018(24)00155-3. Epub 2025 Feb 17. Lancet HIV. 2025. PMID: 39978387 Clinical Trial.
-
Dolutegravir response in antiretroviral therapy naïve and experienced patients with M184V/I: Impact in low-and middle-income settings.Int J Infect Dis. 2021 Apr;105:298-303. doi: 10.1016/j.ijid.2021.03.018. Epub 2021 Mar 16. Int J Infect Dis. 2021. PMID: 33722682
-
Abacavir/dolutegravir/lamivudine single-tablet regimen: a review of its use in HIV-1 infection.Drugs. 2015 Apr;75(5):503-14. doi: 10.1007/s40265-015-0361-6. Drugs. 2015. PMID: 25698454 Review.
-
Incidence and impact of low-level viremia among people living with HIV who received protease inhibitor- or dolutegravir-based antiretroviral therapy.Int J Infect Dis. 2021 Apr;105:147-151. doi: 10.1016/j.ijid.2021.02.045. Epub 2021 Feb 13. Int J Infect Dis. 2021. PMID: 33592339 Clinical Trial.
-
Predictors of treatment-emergent resistance to dolutegravir.Lancet HIV. 2025 Sep;12(9):e660-e663. doi: 10.1016/S2352-3018(25)00127-4. Epub 2025 Jun 19. Lancet HIV. 2025. PMID: 40544855 Review.
Cited by
-
Transitioning to Dolutegravir in a Programmatic Setting: Virological Outcomes and Associated Factors Among Treatment-Naive Patients With HIV-1 in the Kilombero and Ulanga Antiretroviral Cohort in Rural Tanzania.Open Forum Infect Dis. 2023 Jun 26;10(7):ofad321. doi: 10.1093/ofid/ofad321. eCollection 2023 Jul. Open Forum Infect Dis. 2023. PMID: 37520425 Free PMC article.
-
Analytical Assessment of the Vela Diagnostics NGS Assay for HIV Genotyping and Resistance Testing: The Apulian Experience.Int J Mol Sci. 2022 Mar 1;23(5):2727. doi: 10.3390/ijms23052727. Int J Mol Sci. 2022. PMID: 35269868 Free PMC article.
-
Development and Optimization of Oligonucleotide Ligation Assay (OLA) Probes for Detection of HIV-1 Resistance to Dolutegravir.Viruses. 2024 Jul 19;16(7):1162. doi: 10.3390/v16071162. Viruses. 2024. PMID: 39066324 Free PMC article.
-
Mutation patterns of integrase gene affect antiretroviral resistance in various non-B subtypes of human immunodeficiency virus Type-1 and their implications for patients' therapy.Biomedicine (Taipei). 2023 Dec 1;13(4):1-9. doi: 10.37796/2211-8039.1422. eCollection 2023. Biomedicine (Taipei). 2023. PMID: 38532838 Free PMC article. Review.
-
HIV-1 resistance against dolutegravir fluctuates rapidly alongside erratic treatment adherence: a case report.J Glob Antimicrob Resist. 2022 Dec;31:323-327. doi: 10.1016/j.jgar.2022.11.001. Epub 2022 Nov 5. J Glob Antimicrob Resist. 2022. PMID: 36347497 Free PMC article.
References
-
- Saag M.S., Benson C.A., Gandhi R.T., Hoy J.F., Landovitz R.J., Mugavero M.J., Sax P.E., Smith D.M., Thompson M.A., Buchbinder S.P., et al. Antiretroviral drugs for treatment and prevention of HIV Infection in Adults: 2018 recommendations of the international antiviral society-USA panel. JAMA. 2018;320:379–396. doi: 10.1001/jama.2018.8431. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

