Emergence of Resistance to Integrase Strand Transfer Inhibitors during Dolutegravir Containing Triple-Therapy in a Treatment-Experienced Patient with Pre-Existing M184V/I Mutation
- PMID: 33228206
- PMCID: PMC7699495
- DOI: 10.3390/v12111330
Emergence of Resistance to Integrase Strand Transfer Inhibitors during Dolutegravir Containing Triple-Therapy in a Treatment-Experienced Patient with Pre-Existing M184V/I Mutation
Abstract
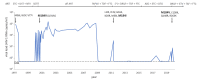
With the current widespread use of dolutegravir in low-income countries, the understanding of the impact of nucleoside reverse transcriptase inhibitor (NRTI-) associated mutations on the efficacy of dolutegravir-containing antiretroviral therapy (ART) is of utmost importance. We describe a rare case of a patient with pre-existing M184V/I mutation and virological failure on a dolutegravir/lamivudine/abacavir regimen with the emergence of integrase strand transfer inhibitor resistance mutations. Additional risk factors, which may have triggered the virological failure, included suboptimal adherence and low nadir CD4+ cell count. This case illustrates that dolutegravir-containing triple-therapy should be prescribed with caution to patients with pre-existing M184V/I mutation and poor efficacy of the reverse transcriptase inhibitor backbone. In addition, this case highlights the need for viral load monitoring in patients on dolutegravir-containing regimens in settings with a high prevalence of the M184V/I mutation such as in low-income countries.
Keywords: HIV-1; M184V/I mutation; antiretroviral therapy; dolutegravir; drug resistance; integrase strand transfer inhibitor; virological failure.
Conflict of interest statement
D.L.B. reports travel grants and honoraria from ViiV, MSD, and Gilead Sciences outside the submitted work. K.J.M. reports grants travel grants and honoraria from ViiV and Gilead Sciences outside the submitted work; the University of Zurich received an unrestricted research grant from Gilead Science for studies that Metzner serves as principal investigator, unrelated to the submitted work. H.F.G. has received unrestricted research grants from Gilead Sciences; fees for data and safety monitoring board or consulting/advisory board membership from Merck Gilead Sciences, ViiV, Sandoz, and Mepha.
Figures
References
-
- Saag M.S., Benson C.A., Gandhi R.T., Hoy J.F., Landovitz R.J., Mugavero M.J., Sax P.E., Smith D.M., Thompson M.A., Buchbinder S.P., et al. Antiretroviral drugs for treatment and prevention of HIV Infection in Adults: 2018 recommendations of the international antiviral society-USA panel. JAMA. 2018;320:379–396. doi: 10.1001/jama.2018.8431. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

