Compound Uptake into E. coli Can Be Facilitated by N-Alkyl Guanidiniums and Pyridiniums
- PMID: 33228356
- PMCID: PMC7796962
- DOI: 10.1021/acsinfecdis.0c00715
Compound Uptake into E. coli Can Be Facilitated by N-Alkyl Guanidiniums and Pyridiniums
Abstract
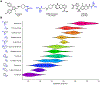
Multidrug-resistant Gram-negative bacterial infections are on the rise, and with no FDA approvals for new classes of broad-spectrum antibiotics in over 50 years, these infections constitute a major threat to human health. A significant challenge is the inability of most compounds to accumulate in Gram-negative bacteria. Recently developed predictive guidelines show that appending a primary amine to an appropriately shaped compound can enhance Gram-negative accumulation. Here, we report that other positively charged nitrogen functional groups, namely, N-alkyl guanidiniums and pyridiniums, can also facilitate compound uptake into Gram-negative bacteria. The accumulation of a set of 60 nonantibiotic compounds, consisting of 20 primary amines and their corresponding guanidiniums and pyridiniums, was assessed in Escherichia coli. We also installed these alternate functional groups onto antibiotic scaffolds and assessed their accumulation and antibacterial activity in Gram-negative bacteria. The results suggest that other positively-charged, nitrogen-containing functional groups should be considered when designing antibiotics with Gram-negative activity.
Keywords: Gram-negative accumulation; amines; antibiotic drug design; guanidiniums; pyridiniums.
Figures

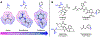

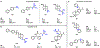


References
-
- CDC (2019) Antibiotic Resistance Threats in the United States, 2019, U.S. Department of Health and Human Services, CDC, Atlanta, GA.
-
- Göttig S, Gruber TM, Higgins PG, Wachsmuth M, Seifert H, and Kempf VAJ (2014) Detection of pan drug-resistant Acinetobacter baumannii in Germany. J. Antimicrob. Chemother 69 (9), 2578–2579. - PubMed
-
- Lewis K (2020) The Science of Antibiotic Discovery. Cell 181 (1), 29–45. - PubMed
-
- Lewis K (2013) Platforms for antibiotic discovery. Nat. Rev. Drug Discovery 12 (5), 371–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

