Coronavirus Disease 2019-Associated Thrombosis and Coagulopathy: Review of the Pathophysiological Characteristics and Implications for Antithrombotic Management
- PMID: 33228447
- PMCID: PMC7955431
- DOI: 10.1161/JAHA.120.019650
Coronavirus Disease 2019-Associated Thrombosis and Coagulopathy: Review of the Pathophysiological Characteristics and Implications for Antithrombotic Management
Abstract
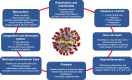
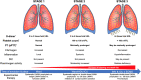
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus-2, which has posed a significant threat to global health. Although the infection is frequently asymptomatic or associated with mild symptoms, in a small proportion of patients it can produce an intense inflammatory and prothrombotic state that can lead to acute respiratory distress syndrome, multiple organ failure, and death. Angiotensin-converting enzyme 2, highly expressed in the respiratory system, has been identified as a functional receptor for severe acute respiratory syndrome coronavirus-2. Notably, angiotensin-converting enzyme 2 is also expressed in the cardiovascular system, and there are multiple cardiovascular implications of COVID-19. Cardiovascular risk factors and cardiovascular disease have been associated with severe manifestations and poor prognosis in patients with COVID-19. More important, patients with COVID-19 may have thrombotic and coagulation abnormalities, promoting a hypercoagulable state and resulting in an increased rate of thrombotic and thromboembolic events. This review will describe the pathophysiological characteristics of the cardiovascular involvement following infection by severe acute respiratory syndrome coronavirus-2, with a focus on thrombotic and thromboembolic manifestations and implications for antithrombotic management.
Keywords: anticoagulant therapy; antiplatelet therapy; coronavirus disease 2019; endothelium; platelets; severe acute respiratory syndrome coronavirus‐2; thrombosis.
Conflict of interest statement
Dr Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol‐Myers Squibb, Chiesi, Daiichi‐Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company; and has received payments for participation in review activities from CeloNova and St Jude Medical. Dr Angiolillo also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi‐Sankyo, Eisai, Eli Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co, Merck, Novartis, Osprey Medical, Renal Guard Solutions, and the Scott R. MacKenzie Foundation. Dr Capodanno declares that he has received consulting and speaker's fees from AstraZeneca, Bayer, Boehringer Ingelheim, and Daiichi Sankyo, outside the present work. Dr Montalescot reports consulting or speaker's fees from Abbott, AIM group, Amgen, Actelion, American College of Cardiology Foundation, AstraZeneca, Axis‐Santé, Bayer, Boston‐Scientific, Bristol‐Myers Squibb, Beth Israel Deaconess Medical, Brigham Women's Hospital, Fréquence Médicale, ICOM, Idorsia, Elsevier, Fédération Française de Cardiologie, Fréquence Médicale, ICAN, Lead‐Up, Menarini, Medtronic, MSD, Novo‐Nordisk, Pfizer, Quantum Genomics, Sanofi‐Aventis, SCOR Global Life, Servier, and WebMD. Dr Ortega‐Paz has no disclosures to report.
Figures



References
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

