Bivalves are NO different: nitric oxide as negative regulator of metamorphosis in the Pacific oyster, Crassostrea gigas
- PMID: 33228520
- PMCID: PMC7686737
- DOI: 10.1186/s12861-020-00232-2
Bivalves are NO different: nitric oxide as negative regulator of metamorphosis in the Pacific oyster, Crassostrea gigas
Abstract
Background: Nitric oxide (NO) is presumed to be a regulator of metamorphosis in many invertebrate species, and although NO pathways have been comparatively well-investigated in gastropods, annelids and crustaceans, there has been very limited research on the effects of NO on metamorphosis in bivalve shellfish.
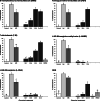
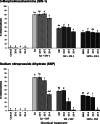
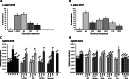
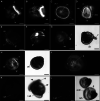
Results: In this paper, we investigate the effects of NO pathway inhibitors and NO donors on metamorphosis induction in larvae of the Pacific oyster, Crassostrea gigas. The nitric oxides synthase (NOS) inhibitors s-methylisothiourea hemisulfate salt (SMIS), aminoguanidine hemisulfate salt (AGH) and 7-nitroindazole (7-NI) induced metamorphosis at 75, 76 and 83% respectively, and operating in a concentration-dependent manner. Additional induction of up to 54% resulted from exposures to 1H-[1,2,4]Oxadiazole[4,3-a]quinoxalin-1-one (ODQ), an inhibitor of soluble guanylyl cyclase, with which NO interacts to catalyse the synthesis of cyclic guanosine monophosphate (cGMP). Conversely, high concentrations of the NO donor sodium nitroprusside dihydrate in combination with metamorphosis inducers epinephrine, MK-801 or SMIS, significantly decreased metamorphosis, although a potential harmful effect of excessive NO unrelated to metamorphosis pathway cannot be excluded. Expression of CgNOS also decreased in larvae after metamorphosis regardless of the inducers used, but intensified again post-metamorphosis in spat. Fluorescent detection of NO in competent larvae with DAF-FM diacetate and localisation of the oyster nitric oxide synthase CgNOS expression by in-situ hybridisation showed that NO occurs primarily in two key larval structures, the velum and foot. cGMP was also detected in the foot using immunofluorescent assays, and is potentially involved in the foot's smooth muscle relaxation.
Conclusion: Together, these results suggest that the NO pathway acts as a negative regulator of metamorphosis in Pacific oyster larvae, and that NO reduction induces metamorphosis by inhibiting swimming or crawling behaviour, in conjunction with a cascade of additional neuroendocrine downstream responses.
Keywords: Bivalves; Crassostrea gigas; Metamorphosis; Nitric oxide; Nitric oxide synthase NOS; Pacific oyster; cGMP.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
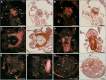
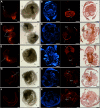
Similar articles
-
The regulatory role of the NO/cGMP signal transduction cascade during larval attachment and metamorphosis of the barnacle Balanus (=Amphibalanus) amphitrite.J Exp Biol. 2012 Nov 1;215(Pt 21):3813-22. doi: 10.1242/jeb.070235. Epub 2012 Aug 1. J Exp Biol. 2012. PMID: 22855617
-
Cloning and characterisation of NMDA receptors in the Pacific oyster, Crassostrea gigas (Thunberg, 1793) in relation to metamorphosis and catecholamine synthesis.Dev Biol. 2021 Jan 1;469:144-159. doi: 10.1016/j.ydbio.2020.10.008. Epub 2020 Oct 22. Dev Biol. 2021. PMID: 33131707
-
Nitric oxide inhibits metamorphosis in larvae of Crepidula fornicata, the slippershell snail.Biol Bull. 2007 Oct;213(2):160-71. doi: 10.2307/25066632. Biol Bull. 2007. PMID: 17928523
-
NO/cGMP signaling and HSP90 activity represses metamorphosis in the sea urchin Lytechinus pictus.Biol Bull. 2001 Dec;201(3):394-404. doi: 10.2307/1543617. Biol Bull. 2001. PMID: 11751251
-
Developmental dynamics of myogenesis in Pacific oyster Crassostrea gigas.Comp Biochem Physiol B Biochem Mol Biol. 2019 Jan;227:21-30. doi: 10.1016/j.cbpb.2018.08.008. Epub 2018 Sep 5. Comp Biochem Physiol B Biochem Mol Biol. 2019. PMID: 30193833
Cited by
-
Stage-Specific Transcriptomes of the Mussel Mytilus coruscus Reveals the Developmental Program for the Planktonic to Benthic Transition.Genes (Basel). 2023 Jan 21;14(2):287. doi: 10.3390/genes14020287. Genes (Basel). 2023. PMID: 36833215 Free PMC article.
-
Expression Pattern of Nitric Oxide Synthase during Development of the Marine Gastropod Mollusc, Crepidula fornicata.Genes (Basel). 2021 Feb 22;12(2):314. doi: 10.3390/genes12020314. Genes (Basel). 2021. PMID: 33671839 Free PMC article.
-
Comparative transcriptomic analysis revealed dynamic changes of distinct classes of genes during development of the Manila clam (Ruditapes philippinarum).BMC Genomics. 2022 Sep 29;23(1):676. doi: 10.1186/s12864-022-08813-0. BMC Genomics. 2022. PMID: 36175832 Free PMC article.
-
The unique biology of catch muscles: insights into structure, function, and robotics innovations.Front Bioeng Biotechnol. 2025 Apr 16;13:1478626. doi: 10.3389/fbioe.2025.1478626. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40309505 Free PMC article. Review.
-
Effects of L-arginine on Nitric Oxide Synthesis and Larval Metamorphosis of Mytilus coruscus.Genes (Basel). 2023 Feb 9;14(2):450. doi: 10.3390/genes14020450. Genes (Basel). 2023. PMID: 36833378 Free PMC article.
References
-
- Gosling E. Reproduction, settlement and recruitment. In: Gosling E, editor. Marine Bivalve Molluscs. 2nd Edition. Chichester: Wiley-Blackwell; 2015. p. 157–202.
-
- Joyce A, Vogeler S. Molluscan bivalve settlement and metamorphosis: neuroendocrine inducers and morphogenetic responses. Aquaculture. 2018;487:64–82. doi: 10.1016/j.aquaculture.2018.01.002. - DOI
-
- Vogeler S, Wikfors GH, Li X, Veilleux D, Miller-Ezzy P, Joyce A. Larval metamorphosis in oyster and clam species in response to NMDA receptor ligands: the NMDA receptor pathway as potential regulator of bivalve’s transition to spat. Aquaculture. 2019;511:634173. doi: 10.1016/j.aquaculture.2019.05.058. - DOI
-
- Vogeler S, Miller-Ezzy P, Li X, Wikfors GH, Joyce A. First report of a putative involvement of the NMDA pathway in Pacific oyster (Crassostrea gigas) development: effect of NMDA receptor ligands on oyster metamorphosis with implications for bivalve hatchery management. Aquaculture. 2018;497:140–146. doi: 10.1016/j.aquaculture.2018.07.048. - DOI
-
- Vogeler S, Carboni S, Li X, Ireland J, Miller-Ezzy P, Joyce A. Cloning and characterisation of NMDA receptors in the Pacific oyster, Crassostrea gigas (Thunberg, 1793) in relation to metamorphosis and catecholamine synthesis. Dev Biol. 2020. 10.1016/j.ydbio.2020.10.008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

