Rhamnolipids and surfactin inhibit the growth or formation of oral bacterial biofilm
- PMID: 33228524
- PMCID: PMC7684882
- DOI: 10.1186/s12866-020-02034-9
Rhamnolipids and surfactin inhibit the growth or formation of oral bacterial biofilm
Abstract
Background: Bacteria survive in various environments by forming biofilms. Bacterial biofilms often cause significant problems to medical instruments and industrial processes. Techniques to inhibit biofilm formation are essential and have wide applications. In this study, we evaluated the ability of two types of biosurfactants (rhamnolipids and surfactin) to inhibit growth and biofilm formation ability of oral pathogenic bacteria such as Aggregatibacter actinomycetemcomitans, Streptococcus mutans, and Streptococcus sanguinis.
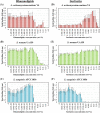
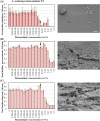
Results: Rhamnolipids inhibited the growth and biofilm formation ability of all examined oral bacteria. Surfactin showed effective inhibition against S. sanguinis ATCC10556, but lower effects toward A. actinomycetemcomitans Y4 and S. mutans UA159. To corroborate these results, biofilms were observed by scanning electron microscopy (SEM) and confocal microscopy. The observations were largely in concordance with the biofilm assay results. We also attempted to determine the step in the biofilm formation process that was inhibited by biosurfactants. The results clearly demonstrated that rhamnolipids inhibit biofilm formation after the initiation process, however, they do not affect attachment or maturation.
Conclusions: Rhamnolipids inhibit oral bacterial growth and biofilm formation by A. actinomycetemcomitans Y4, and may serve as novel oral drug against localized invasive periodontitis.
Keywords: Biofilm inhibition; Oral bacteria; Rhamnolipids; Surfactin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Rhamnolipids from non-pathogenic Burkholderia thailandensis E264: Physicochemical characterization, antimicrobial and antibiofilm efficacy against oral hygiene related pathogens.N Biotechnol. 2017 May 25;36:26-36. doi: 10.1016/j.nbt.2016.12.009. Epub 2017 Jan 5. N Biotechnol. 2017. PMID: 28065676
-
Rhamnolipids and lactonic sophorolipids: natural antimicrobial surfactants for oral hygiene.J Appl Microbiol. 2017 Nov;123(5):1111-1123. doi: 10.1111/jam.13550. Epub 2017 Oct 16. J Appl Microbiol. 2017. PMID: 28766815
-
Effect of arginine on the growth and biofilm formation of oral bacteria.Arch Oral Biol. 2017 Oct;82:256-262. doi: 10.1016/j.archoralbio.2017.06.026. Epub 2017 Jun 24. Arch Oral Biol. 2017. PMID: 28668766
-
The involvement of rhamnolipids in microbial cell adhesion and biofilm development - an approach for control?Lett Appl Microbiol. 2014 May;58(5):447-53. doi: 10.1111/lam.12211. Epub 2014 Jan 24. Lett Appl Microbiol. 2014. PMID: 24372465 Review.
-
Nature's Marvels: Exploring the Multifaceted Applications of Surfactin and Rhamnolipids.Langmuir. 2025 Feb 18;41(6):3731-3743. doi: 10.1021/acs.langmuir.4c04093. Epub 2025 Feb 9. Langmuir. 2025. PMID: 39924911 Review.
Cited by
-
Candida krusei M4CK Produces a Bioemulsifier That Acts on Melaleuca Essential Oil and Aids in Its Antibacterial and Antibiofilm Activity.Antibiotics (Basel). 2023 Nov 30;12(12):1686. doi: 10.3390/antibiotics12121686. Antibiotics (Basel). 2023. PMID: 38136720 Free PMC article.
-
[Effect of hypochloric acid on Escherichia coli biofilm and the clinical efficacy of hypochloric acid for wounds with Escherichia coli infection].Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022 Mar 20;38(3):242-250. doi: 10.3760/cma.j.cn501120-20201112-00471. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022. PMID: 35325969 Free PMC article. Clinical Trial. Chinese.
-
Intestinal biofilms: pathophysiological relevance, host defense, and therapeutic opportunities.Clin Microbiol Rev. 2024 Sep 12;37(3):e0013323. doi: 10.1128/cmr.00133-23. Epub 2024 Jul 12. Clin Microbiol Rev. 2024. PMID: 38995034 Free PMC article. Review.
-
Surface-Active Compounds Produced by Microorganisms: Promising Molecules for the Development of Antimicrobial, Anti-Inflammatory, and Healing Agents.Antibiotics (Basel). 2022 Aug 16;11(8):1106. doi: 10.3390/antibiotics11081106. Antibiotics (Basel). 2022. PMID: 36009975 Free PMC article. Review.
-
Bacteriophage-Derived Depolymerases against Bacterial Biofilm.Antibiotics (Basel). 2021 Feb 10;10(2):175. doi: 10.3390/antibiotics10020175. Antibiotics (Basel). 2021. PMID: 33578658 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

