Mice lacking global Stap1 expression do not manifest hypercholesterolemia
- PMID: 33228548
- PMCID: PMC7685646
- DOI: 10.1186/s12881-020-01176-x
Mice lacking global Stap1 expression do not manifest hypercholesterolemia
Abstract
Background: Autosomal dominant familial hypercholesterolemia (ADH; MIM#143890) is one of the most common monogenic disorders characterized by elevated circulatory LDL cholesterol. Initial studies in humans with ADH identified a potential relationship with variants of the gene encoding signal transducing adaptor family member protein 1 (STAP1; MIM#604298). However, subsequent studies have been contradictory. In this study, mice lacking global Stap1 expression (Stap1-/-) were characterized under standard chow and a 42% kcal western diet (WD).
Methods: Mice were studied for changes in different metabolic parameters before and after a 16-week WD regime. Growth curves, body fats, circulatory lipids, parameters of glucose homeostasis, and liver architecture were studied for comparisons.
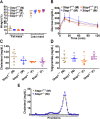
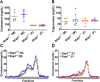
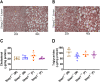
Results: Surprisingly, Stap1-/- mice fed the 16-week WD demonstrated no marked differences in any of the metabolic parameters compared to Stap1+/+ mice. Furthermore, hepatic architecture and cholesterol content in FPLC-isolated lipoprotein fractions also remained comparable to wild-type mice.
Conclusion: These results strongly suggest that STAP1 does not alter lipid levels, that a western diet did not exacerbate a lipid disorder in Stap1 deficient mice and support the contention that it is not causative for hyperlipidemia in ADH patients. These results support other published studies also questioning the role of this locus in human hypercholesterolemia.
Keywords: Autosomal dominant familial hypercholesterolemia; B-cells; Familial hypercholesterolemia 4; Fast performance liquid chromatography; STAP1; Western diet.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Ezhov M, Bazhan S, Ershova A. Clinical guidelines for familial hypercholesterolemia. J Atherosclerosis Dyslipidemias. 2019;1:5–43.
-
- Goldberg AC, Hopkins PN, Toth PP, Ballantyne CM, Rader DJ, Robinson JG, Daniels SR, Gidding SS, de Ferranti SD, Ito MK, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on familial hypercholesterolemia. J Clin Lipidol. 2011;5(3 Suppl):S1–S8. doi: 10.1016/j.jacl.2011.04.003. - DOI - PubMed
-
- Gidding SS, Champagne MA, de Ferranti SD, Defesche J, Ito MK, Knowles JW, McCrindle B, Raal F, Rader D, Santos RD, et al. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132(22):2167–2192. doi: 10.1161/CIR.0000000000000297. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

