A cassava protoplast system for screening genes associated with the response to South African cassava mosaic virus
- PMID: 33228712
- PMCID: PMC7685591
- DOI: 10.1186/s12985-020-01453-4
A cassava protoplast system for screening genes associated with the response to South African cassava mosaic virus
Abstract
Background: The study of transient gene expression in cassava plants during virus infection using existing protocols is laborious and may take approximately fifteen weeks due to cassava's recalcitrance to transformation. The combination of a protoplast system with CRISPR-mediated gene editing promises to shorten the turnaround time from plant tissue culture to high-throughput gene expression screening for candidate genes. Here, we detail a protocol for screening genes associated with the response to South African cassava mosaic virus (SACMV) in cassava protoplasts, with reference to the ubiquitin E3 ligase gene, MeE3L.
Methods: Cassava protoplasts of model, and SACMV-susceptible and -tolerant genotypes, were transformed with SACMV infectious clones and/or a CRISPR-editing construct targeting the MeE3L using PEG4000-mediated transfection. DNA and RNA were extracted from transformed protoplasts at 24 h post-transfection. Relative SACMV DNA accumulation was determined via qPCR using DpnI-digested total DNA, MeE3L relative expression was determined via reverse transcriptase qPCR, and results were analysed using one-way ANOVA, Tukey's HSD test and the 2-ΔΔCTstatistical method. The MeE3L exonic region was sequenced on the ABI 3500XL Genetic Analyzer platform; and sequences were analysed for mutations using MAFTT and MEGA-X software. Construction of a phylogenetic tree was done using the Maximum Likelihood method and Jones-Taylor-Thornton (JTT) matrix-based model.
Results: The differential expression of unedited and mutant MeE3L during SACMV infection of model, susceptible and tolerant cassava protoplasts was determined within 7 weeks after commencement of tissue culture. The study also revealed that SACMV DNA accumulation in cassava protoplasts is genotype-dependent and induces multiple mutations in the tolerant landrace MeE3L homolog. Notably, the susceptible cassava landrace encodes a RINGless MeE3Lwhich is silenced by SACMV-induced mutations. SACMV also induces mutations which silence the MeE3L RING domain in protoplasts from and tolerant cassava landraces.
Conclusions: This protocol presented here halves the turnaround time for high-throughput screening of genes associated with the host response to SACMV. It provides evidence that a cassava E3 ligase is associated with the response to SACMV and forms a basis for validation of these findings by in planta functional and interaction studies.
Keywords: Cassava mosaic disease; Geminivirus; Protoplast, ubiquitin E3 ligase.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

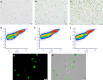

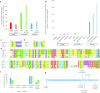
Similar articles
-
A Coiled-Coil Nucleotide-Binding Domain Leucine-Rich Repeat Receptor Gene MeRPPL1 Plays a Role in the Replication of a Geminivirus in Cassava.Viruses. 2024 Jun 11;16(6):941. doi: 10.3390/v16060941. Viruses. 2024. PMID: 38932233 Free PMC article.
-
Small RNA and methylation responses in susceptible and tolerant landraces of cassava infected with South African cassava mosaic virus.Virus Res. 2016 Oct 2;225:10-22. doi: 10.1016/j.virusres.2016.08.011. Epub 2016 Aug 29. Virus Res. 2016. PMID: 27586073
-
Comparative analysis of pattern-triggered and effector-triggered immunity gene expression in susceptible and tolerant cassava genotypes following begomovirus infection.PLoS One. 2025 Jun 4;20(6):e0318442. doi: 10.1371/journal.pone.0318442. eCollection 2025. PLoS One. 2025. PMID: 40465802 Free PMC article.
-
Cassava Mosaic and Brown Streak Diseases: Current Perspectives and Beyond.Annu Rev Virol. 2017 Sep 29;4(1):429-452. doi: 10.1146/annurev-virology-101416-041913. Epub 2017 Jun 23. Annu Rev Virol. 2017. PMID: 28645239 Review.
-
Cassava mosaic geminiviruses in Africa.Plant Mol Biol. 2004 Nov;56(4):585-99. doi: 10.1007/s11103-004-1651-7. Plant Mol Biol. 2004. PMID: 15630622 Review.
Cited by
-
WRKY Transcription Factors in Cassava Contribute to Regulation of Tolerance and Susceptibility to Cassava Mosaic Disease through Stress Responses.Viruses. 2021 Sep 13;13(9):1820. doi: 10.3390/v13091820. Viruses. 2021. PMID: 34578401 Free PMC article.
-
An efficient method for protoplast-mediated production of transformed castor bean (Ricinus communis) lines.BMC Res Notes. 2023 Jul 6;16(1):140. doi: 10.1186/s13104-023-06414-y. BMC Res Notes. 2023. PMID: 37415245 Free PMC article.
-
Automated, High-Throughput Protoplast Transfection for Gene Editing and Transgene Expression Studies.Methods Mol Biol. 2023;2653:129-149. doi: 10.1007/978-1-0716-3131-7_9. Methods Mol Biol. 2023. PMID: 36995624
-
A Coiled-Coil Nucleotide-Binding Domain Leucine-Rich Repeat Receptor Gene MeRPPL1 Plays a Role in the Replication of a Geminivirus in Cassava.Viruses. 2024 Jun 11;16(6):941. doi: 10.3390/v16060941. Viruses. 2024. PMID: 38932233 Free PMC article.
-
Genetic amelioration of fruit and vegetable crops to increase biotic and abiotic stress resistance through CRISPR Genome Editing.Front Plant Sci. 2023 Sep 29;14:1260102. doi: 10.3389/fpls.2023.1260102. eCollection 2023. Front Plant Sci. 2023. PMID: 37841604 Free PMC article. Review.
References
-
- Alabi OJ, Kumar LP, Naidu RA. Cassava Mosaic Disease: A curse to food security in Sub-Saharan Afrca. APSnetFeatur. 2011;
-
- Allie F, Pierce EJ, Okoniewski MJ, Rey C. Transcriptional analysis of South African cassava mosaic virus-infected susceptible and tolerant landraces of cassava highlights differences in resistance, basal defense and cell wall associated genes during infection. BMC Genom. 2014;15(1):1006. doi: 10.1186/1471-2164-15-1006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

