Focal loss of the paranodal domain protein Neurofascin155 in the internal capsule impairs cortically induced muscle activity in vivo
- PMID: 33228720
- PMCID: PMC7685608
- DOI: 10.1186/s13041-020-00698-y
Focal loss of the paranodal domain protein Neurofascin155 in the internal capsule impairs cortically induced muscle activity in vivo
Abstract
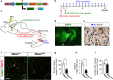
Paranodal axoglial junctions are essential for rapid nerve conduction and the organization of axonal domains in myelinated axons. Neurofascin155 (Nfasc155) is a glial cell adhesion molecule that is also required for the assembly of these domains. Previous studies have demonstrated that general ablation of Nfasc155 disorganizes these domains, reduces conduction velocity, and disrupts motor behaviors. Multiple sclerosis (MS), a typical disorder of demyelination in the central nervous system, is reported to have autoantibody to Nfasc. However, the impact of focal loss of Nfasc155, which may occur in MS patients, remains unclear. Here, we examined whether restricted focal loss of Nfasc155 affects the electrophysiological properties of the motor system in vivo. Adeno-associated virus type5 (AAV5) harboring EGFP-2A-Cre was injected into the glial-enriched internal capsule of floxed-Neurofascin (NfascFlox/Flox) mice to focally disrupt paranodal junctions in the cortico-fugal fibers from the motor cortex to the spinal cord. Electromyograms (EMGs) of the triceps brachii muscles in response to electrical stimulation of the motor cortex were successively examined in these awake mice. EMG analysis showed significant delay in the onset and peak latencies after AAV injection compared to control (Nfasc+/+) mice. Moreover, EMG half-widths were increased, and EMG amplitudes were gradually decreased by 13 weeks. Similar EMG changes have been reported in MS patients. These findings provide physiological evidence that motor outputs are obstructed by focal ablation of paranodal junctions in myelinated axons. Our findings may open a new path toward development of a novel biomarker for an early phase of human MS, as Nfasc155 detects microstructural changes in the paranodal junction.
Keywords: Electromyogram; Motor system; Multiple sclerosis; Neurofascin155; Paranodal junction.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/S0896-6273(01)00294-X. - DOI - PubMed
-
- Calabrese M, Agosta F, Rinaldi F, Mattisi I, Grossi P, Favaretto A, Atzori M, Bernardi V, Barachino L, Rinaldi L, Perini P, Gallo P, Filippi M. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol. 2009;66:1144–1150. doi: 10.1001/archneurol.2009.174. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

