Hemoperfusion with HA380 in acute type A aortic dissection patients undergoing aortic arch operation (HPAO): a randomized, controlled, double-blind clinical trial
- PMID: 33228727
- PMCID: PMC7684885
- DOI: 10.1186/s13063-020-04858-2
Hemoperfusion with HA380 in acute type A aortic dissection patients undergoing aortic arch operation (HPAO): a randomized, controlled, double-blind clinical trial
Abstract
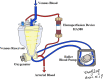
Background: Cardiopulmonary bypass (CPB) is an important cause of significant systemic inflammatory response syndrome (SIRS) in the surgical treatment of acute type A aortic dissection (ATAAD). In patients with arch vessel involvement, extensive surgical repairs often necessitate prolonged use of CPB and results in extensive inflammatory responses. Cytokines and chemokines released during CPB contribute to the progression of SIRS, increase perioperative complications, and negatively impact surgical outcomes. A cytokine adsorber (HA380) is expected to reduce the level of cytokines during CPB, which may decrease both intraoperative and postoperative inflammation. The purpose of this study is to investigate if HA380 is able to reduce the levels of inflammatory cytokines and decrease perioperative complications in ATAAD patients undergoing CPB and deep hypothermic circulatory arrest (DHCA).
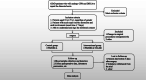
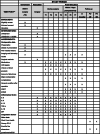
Methods: This study is a single-center, randomized, controlled, double-blind clinical trial. The study aims to recruit 88 patients with ATAAD and aortic arch involvement who will undergo CPB and DHCA to repair the dissected aorta. Patients will be randomized equally into the CPB/DHCA only group (control group) and the CPB/DHCA + HA380 hemoperfusion group (intervention group), with 44 patients each. Patients in the control group will undergo CPB and DHCA only, while patients in the intervention group will undergo continuous hemoperfusion with HA380, in addition to CPB and DHCA. The primary outcome is a composite of major perioperative complications. The secondary outcomes include related inflammatory markers, coagulation parameters, and minor perioperative complications. To comprehensively evaluate the effect of hemoperfusion on the perioperative outcomes, we will also determine if there are differences in perioperative all-cause mortality, length of ICU stay, and total hospitalization costs.
Discussion: In the current trial, hemoperfusion will be applied in patients undergoing CPB and DHCA for repair of the aorta involving the aortic arch. This trial aims to test the safety and efficacy of our hemoperfusion device (HA380) in such settings. Upon completion of the trial, we will determine if HA380 is effective in reducing perioperative proinflammatory cytokine levels. Further, we will also verify if reduction in the proinflammatory cytokine levels, if present, translates to improvement in patient outcomes.
Trial registration: ClinicalTrials.gov NCT04007484 . Registered on 1 July 2019 (retrospectively registered).
Keywords: Acute type A aortic dissection; Cardiopulmonary bypass; Coagulation indicators; Deep hypothermic circulatory arrest; HA380; Inflammatory factors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

