SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis
- PMID: 33228974
- PMCID: PMC7584920
- DOI: 10.1016/j.cmi.2020.10.020
SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis
Abstract
Objectives: COVID-19 has been arguably the most important public health concern worldwide in 2020, and efforts are now escalating to suppress or eliminate its spread. In this study we undertook a meta-analysis to estimate the global and regional seroprevalence rates in humans of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and to assess whether seroprevalence is associated with geographical, climatic and/or sociodemographic factors.
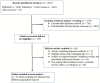
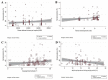
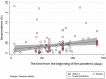
Methods: We systematically reviewed PubMed, Scopus, Embase, medRxiv and bioRxiv databases for preprints or peer-reviewed articles (up to 14 August 2020). Study eligibility criteria were population-based studies describing the prevalence of anti-SARS-CoV-2 (IgG and/or IgM) serum antibodies. Participants were people from different socioeconomic and ethnic backgrounds (from the general population), whose prior COVID-19 status was unknown and who were tested for the presence of anti-SARS-CoV-2 serum antibodies. We used a random-effects model to estimate pooled seroprevalence, and then extrapolated the findings to the global population (for 2020). Subgroup and meta-regression analyses explored potential sources of heterogeneity in the data, and relationships between seroprevalence and sociodemographic, geographical and/or climatic factors.
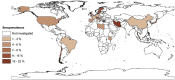
Results: In total, 47 studies involving 399 265 people from 23 countries met the inclusion criteria. Heterogeneity (I2 = 99.4%, p < 0.001) was seen among studies; SARS-CoV-2 seroprevalence in the general population varied from 0.37% to 22.1%, with a pooled estimate of 3.38% (95%CI 3.05-3.72%; 15 879/399 265). On a regional level, seroprevalence varied from 1.45% (0.95-1.94%, South America) to 5.27% (3.97-6.57%, Northern Europe), although some variation appeared to relate to the serological assay used. The findings suggested an association of seroprevalence with income levels, human development indices, geographic latitudes and/or climate. Extrapolating to the 2020 world population, we estimated that 263.5 million individuals had been exposed or infected at the time of this study.
Conclusions: This study showed that SARS-CoV-2 seroprevalence varied markedly among geographic regions, as might be expected early in a pandemic. Longitudinal surveys to continually monitor seroprevalence around the globe will be critical to support prevention and control efforts, and might indicate levels of endemic stability or instability in particular countries and regions.
Keywords: COVID-19; General population; Global seroprevalence; Meta-analysis; SARS-CoV-2; Serum antibodies (IgG and/or IgM); Subgroup analyses.
Copyright © 2020 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures
References
-
- Hick J.L., Biddinger P.D. Novel coronavirus and old lessons—preparing the health system for the pandemic. N Engl J Med. 2020;382:e55. - PubMed
-
- World Health Organization (WHO) Coronavirus disease 2019 (COVID-19) Situation Report—209. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2... Available from:
-
- Chan J.F.-W., Yip C.C.-Y., To K.K.-W., Tang T.H.-C., Wong S.C.-Y., Leung K.-H. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020;58:e00310–e00320. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

