Efficacy and safety of rilonacept for recurrent pericarditis: results from a phase II clinical trial
- PMID: 33229362
- PMCID: PMC7925818
- DOI: 10.1136/heartjnl-2020-317928
Efficacy and safety of rilonacept for recurrent pericarditis: results from a phase II clinical trial
Abstract
Objective: Recurrent pericarditis (RP) incurs significant morbidity. Rilonacept inhibits both interleukin-1 alpha (IL-1α) and IL-1β; these cytokines are thought to play a major role in RP. This phase II study evaluated rilonacept efficacy and safety in RP.
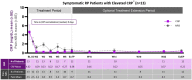
Methods: This multicentre, open-label study enrolled adult patients with idiopathic or postpericardiotomy RP, symptomatic (≥2 pericarditis recurrences) or corticosteroid (CS) dependent (≥2 recurrences prior).Patients received rilonacept 320 mg SC load/160 mg SC weekly maintenance in a 6-week base treatment period (TP) followed by an optional 18-week on-treatment extension period (EP) (option to wean background therapy).
Results: Outcomes: pericarditis pain (numeric rating scale (NRS)) and inflammation (C reactive protein (CRP)) for symptomatic patients; disease activity after CS taper for CS-dependent patients.
Secondary outcomes: health-related quality of life (HRQOL), pericarditis manifestations and additional medications. 25 unique patients enrolled, while 23 completed the EP (seven colchicine failures and five CS failures). In symptomatic patients, NRS and CRP decreased; response was observed after first rilonacept dose. NRS decreased from 4.5 at baseline to 0.7, and CRP decreased from 4.62 mg/dL at baseline to 0.38 mg/dL at end of TP. Median time to CRP normalisation: 9 days. Pericarditis manifestations resolved. 13 patients on CS at baseline completed the EP; 11 (84.6%) discontinued CS, and 2 tapered; CRP and NRS remained low without recurrence. Mean HRQOL scores improved in symptomatic patients. One serious adverse event (SAE) resulted in discontinuation of rilonacept.
Conclusions: Rilonacept led to rapid and sustained improvement in pain, inflammation (CRP and pericarditis manifestations) and HRQOL. CSs were successfully tapered or discontinued; safety was consistent with known rilonacept safety profile.
Trial registration number: NCT03980522.
Keywords: inflammatory markers; pericardial disease; pericarditis.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: ALK: research grant, scientific advisory board Kiniksa Pharmaceuticals, Ltd; advisory board Swedish Orphan Biovitrum AB, advisory board Pfizer, Inc. PCC: advisory board Swedish Orphan Biovitrum AB; advisory board Kiniksa Pharmaceuticals, Ltd. SAL: honoraria – advisory board member for Kiniksa Pharmaceuticals, Ltd; consultant and advisory board member for Swedish Orphan Biovitrum AB. AA: research grants from Kiniksa Pharmaceuticals, Ltd, Swedish Orphan Biovitrum AB, Olatec Therapeutics LLC, Serpin Pharma, LLC; consultant fees: Kiniksa Pharmaceuticals, Ltd, Olatec Therapeutics LLC, Serpin Pharma, LLC and Merck & Co, Inc. ML: one seminar for Kiniksa Pharmaceuticals, Ltd. AB: Kiniksa Pharmaceuticals, Ltd consultant. FF: Kiniksa Pharmaceuticals Corp employee. JFP: Kiniksa Pharmaceuticals Corp employee.
Figures
References
-
- Adler Y, Charron P, Imazio M, et al. . 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921–64. 10.1093/eurheartj/ehv318 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous