The Invariant NKT Cell Response Has Differential Signaling Requirements during Antigen-Dependent and Antigen-Independent Activation
- PMID: 33229442
- PMCID: PMC7855310
- DOI: 10.4049/jimmunol.2000870
The Invariant NKT Cell Response Has Differential Signaling Requirements during Antigen-Dependent and Antigen-Independent Activation
Abstract
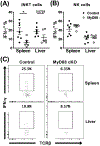
Invariant NKT (iNKT) cells are an innate-like population characterized by their recognition of glycolipid Ags and rapid cytokine production upon activation. Unlike conventional T cells, which require TCR ligation, iNKT cells can also be stimulated independently of their TCR. This feature allows iNKT cells to respond even in the absence of glycolipid Ags, for example, during viral infections. Although the TCR-dependent and -independent activation of iNKT cells have been relatively well established, the exact contributions of IL-12, IL-18, and TLRs remain unclear for these two activation pathways. To definitively investigate how these components affect the direct and indirect stimulation of iNKT cells, we used mice deficient for either MyD88 or the IL-12Rβ2 in the T cell lineage. Using these tools, we demonstrate that IL-12, IL-18, and TLRs are completely dispensable for the TCR activation pathway when a strong agonist is used. In contrast, during murine CMV infection, when the TCR is not engaged, IL-12 signaling is essential, and TLR signaling is expendable. Importantly, to our knowledge, we discovered an intrinsic requirement for IL-18 signaling by splenic iNKT cells but not liver iNKT cells, suggesting that there might be diversity, even within the NKT1 population.
Copyright © 2020 by The American Association of Immunologists, Inc.
Figures






References
-
- Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, and Brenner MB. 1994. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature 372: 691–694. - PubMed
-
- Crowe NY, Uldrich AP, Kyparissoudis K, Hammond KJ, Hayakawa Y, Sidobre S, Keating R, Kronenberg M, Smyth MJ, and Godfrey DI. 2003. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. Journal of immunology (Baltimore, Md. : 1950) 171: 4020–4027. - PubMed
-
- Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang CR, Joyce S, Wick MJ, and Van Kaer L. 2003. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proceedings of the National Academy of Sciences of the United States of America 100: 10913–10918. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

