Post-intervention Status in Patients With Refractory Myasthenia Gravis Treated With Eculizumab During REGAIN and Its Open-Label Extension
- PMID: 33229455
- PMCID: PMC7905790
- DOI: 10.1212/WNL.0000000000011207
Post-intervention Status in Patients With Refractory Myasthenia Gravis Treated With Eculizumab During REGAIN and Its Open-Label Extension
Abstract
Objective: To evaluate whether eculizumab helps patients with anti-acetylcholine receptor-positive (AChR+) refractory generalized myasthenia gravis (gMG) achieve the Myasthenia Gravis Foundation of America (MGFA) post-intervention status of minimal manifestations (MM), we assessed patients' status throughout REGAIN (Safety and Efficacy of Eculizumab in AChR+ Refractory Generalized Myasthenia Gravis) and its open-label extension.
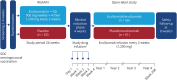
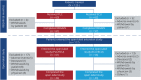
Methods: Patients who completed the REGAIN randomized controlled trial and continued into the open-label extension were included in this tertiary endpoint analysis. Patients were assessed for the MGFA post-intervention status of improved, unchanged, worse, MM, and pharmacologic remission at defined time points during REGAIN and through week 130 of the open-label study.
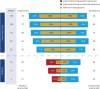
Results: A total of 117 patients completed REGAIN and continued into the open-label study (eculizumab/eculizumab: 56; placebo/eculizumab: 61). At week 26 of REGAIN, more eculizumab-treated patients than placebo-treated patients achieved a status of improved (60.7% vs 41.7%) or MM (25.0% vs 13.3%; common OR: 2.3; 95% CI: 1.1-4.5). After 130 weeks of eculizumab treatment, 88.0% of patients achieved improved status and 57.3% of patients achieved MM status. The safety profile of eculizumab was consistent with its known profile and no new safety signals were detected.
Conclusion: Eculizumab led to rapid and sustained achievement of MM in patients with AChR+ refractory gMG. These findings support the use of eculizumab in this previously difficult-to-treat patient population.
Clinicaltrialsgov identifier: REGAIN, NCT01997229; REGAIN open-label extension, NCT02301624.
Classification of evidence: This study provides Class II evidence that, after 26 weeks of eculizumab treatment, 25.0% of adults with AChR+ refractory gMG achieved MM, compared with 13.3% who received placebo.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
-
- Silvestri NJ, Wolfe GI. Treatment-refractory myasthenia gravis. J Clin Neuromuscul Dis 2014;15:167–178. - PubMed
-
- Engel-Nitz NM, Boscoe A, Wolbeck R, Johnson J, Silvestri NJ. Burden of illness in patients with treatment refractory myasthenia gravis. Muscle Nerve 2018;58:99–105. - PubMed
-
- Murai H. Japanese clinical guidelines for myasthenia gravis: putting into practice. Clin Exp Neuroimmunol 2015;6:21–31.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
