Studies in bank voles reveal strain differences between chronic wasting disease prions from Norway and North America
- PMID: 33229531
- PMCID: PMC7733848
- DOI: 10.1073/pnas.2013237117
Studies in bank voles reveal strain differences between chronic wasting disease prions from Norway and North America
Abstract
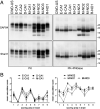
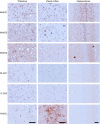
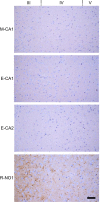
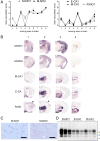
Chronic wasting disease (CWD) is a relentless epidemic disorder caused by infectious prions that threatens the survival of cervid populations and raises increasing public health concerns in North America. In Europe, CWD was detected for the first time in wild Norwegian reindeer (Rangifer tarandus) and moose (Alces alces) in 2016. In this study, we aimed at comparing the strain properties of CWD prions derived from different cervid species in Norway and North America. Using a classical strain typing approach involving transmission and adaptation to bank voles (Myodes glareolus), we found that prions causing CWD in Norway induced incubation times, neuropathology, regional deposition of misfolded prion protein aggregates in the brain, and size of their protease-resistant core, different from those that characterize North American CWD. These findings show that CWD prion strains affecting Norwegian cervids are distinct from those found in North America, implying that the highly contagious North American CWD prions are not the proximate cause of the newly discovered Norwegian CWD cases. In addition, Norwegian CWD isolates showed an unexpected strain variability, with reindeer and moose being caused by different CWD strains. Our findings shed light on the origin of emergent European CWD, have significant implications for understanding the nature and the ecology of CWD in Europe, and highlight the need to assess the zoonotic potential of the new CWD strains detected in Europe.
Keywords: CWD; TSE; chronic wasting disease; prion strains; prions.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Ironside J. W., Ritchie D. L., Head M. W., Prion diseases. Handb. Clin. Neurol. 145, 393–403 (2017). - PubMed
-
- Knight R., Infectious and sporadic prion diseases. Prog. Mol. Biol. Transl. Sci. 150, 293–318 (2017). - PubMed
-
- Kim T. Y., et al., Additional cases of chronic wasting disease in imported deer in Korea. J. Vet. Med. Sci. 67, 753–759 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

