Therapeutic candidates for the Zika virus identified by a high-throughput screen for Zika protease inhibitors
- PMID: 33229545
- PMCID: PMC7733812
- DOI: 10.1073/pnas.2005463117
Therapeutic candidates for the Zika virus identified by a high-throughput screen for Zika protease inhibitors
Abstract
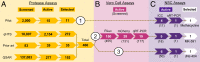
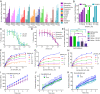
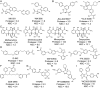
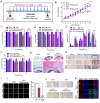
When Zika virus emerged as a public health emergency there were no drugs or vaccines approved for its prevention or treatment. We used a high-throughput screen for Zika virus protease inhibitors to identify several inhibitors of Zika virus infection. We expressed the NS2B-NS3 Zika virus protease and conducted a biochemical screen for small-molecule inhibitors. A quantitative structure-activity relationship model was employed to virtually screen ∼138,000 compounds, which increased the identification of active compounds, while decreasing screening time and resources. Candidate inhibitors were validated in several viral infection assays. Small molecules with favorable clinical profiles, especially the five-lipoxygenase-activating protein inhibitor, MK-591, inhibited the Zika virus protease and infection in neural stem cells. Members of the tetracycline family of antibiotics were more potent inhibitors of Zika virus infection than the protease, suggesting they may have multiple mechanisms of action. The most potent tetracycline, methacycline, reduced the amount of Zika virus present in the brain and the severity of Zika virus-induced motor deficits in an immunocompetent mouse model. As Food and Drug Administration-approved drugs, the tetracyclines could be quickly translated to the clinic. The compounds identified through our screening paradigm have the potential to be used as prophylactics for patients traveling to endemic regions or for the treatment of the neurological complications of Zika virus infection.
Keywords: Zika virus; encephalitis; flavivirus; high-throughput screening; serine protease.
Conflict of interest statement
The authors declare no competing interest.
Figures
References
-
- Mlakar J., et al. , Zika virus associated with microcephaly. N. Engl. J. Med. 374, 951–958 (2016). - PubMed
-
- Carod-Artal F. J., Neurological complications of Zika virus infection. Expert Rev. Anti Infect. Ther. 16, 399–410 (2018). - PubMed
-
- Brady O. J., Hay S. I., The first local cases of Zika virus in Europe. Lancet 394, 1991–1992 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

