Cellular pathways of calcium transport and concentration toward mineral formation in sea urchin larvae
- PMID: 33229583
- PMCID: PMC7733801
- DOI: 10.1073/pnas.1918195117
Cellular pathways of calcium transport and concentration toward mineral formation in sea urchin larvae
Abstract
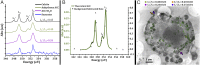
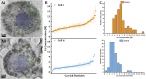
Sea urchin larvae have an endoskeleton consisting of two calcitic spicules. The primary mesenchyme cells (PMCs) are the cells that are responsible for spicule formation. PMCs endocytose sea water from the larval internal body cavity into a network of vacuoles and vesicles, where calcium ions are concentrated until they precipitate in the form of amorphous calcium carbonate (ACC). The mineral is subsequently transferred to the syncytium, where the spicule forms. Using cryo-soft X-ray microscopy we imaged intracellular calcium-containing particles in the PMCs and acquired Ca-L2,3 X-ray absorption near-edge spectra of these Ca-rich particles. Using the prepeak/main peak (L2'/ L2) intensity ratio, which reflects the atomic order in the first Ca coordination shell, we determined the state of the calcium ions in each particle. The concentration of Ca in each of the particles was also determined by the integrated area in the main Ca absorption peak. We observed about 700 Ca-rich particles with order parameters, L2'/ L2, ranging from solution to hydrated and anhydrous ACC, and with concentrations ranging between 1 and 15 M. We conclude that in each cell the calcium ions exist in a continuum of states. This implies that most, but not all, water is expelled from the particles. This cellular process of calcium concentration may represent a widespread pathway in mineralizing organisms.
Keywords: ACC; XAS; biomineralization; cryo-SXM; spectromicroscopy.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Carafoli E., Calcium–A universal carrier of biological signals. Delivered on 3 July 2003 at the special FEBS meeting in Brussels. FEBS J. 272, 1073–1089 (2005). - PubMed
-
- Berridge M. J., Lipp P., Bootman M. D., The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21 (2000). - PubMed
-
- Gangola P., Rosen B. P., Maintenance of intracellular calcium in Escherichia coli. J. Biol. Chem. 262, 12570–12574 (1987). - PubMed
-
- Batiza A. F., Schulz T., Masson P. H., Yeast respond to hypotonic shock with a calcium pulse. J. Biol. Chem. 271, 23357–23362 (1996). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

