A forced swim-based rat model of premenstrual depression: effects of hormonal changes and drug intervention
- PMID: 33229622
- PMCID: PMC7762461
- DOI: 10.18632/aging.202249
A forced swim-based rat model of premenstrual depression: effects of hormonal changes and drug intervention
Abstract
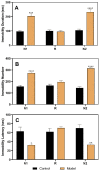
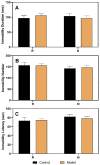
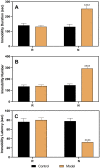
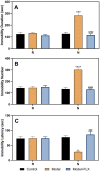
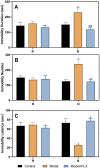
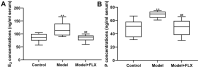
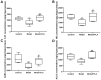
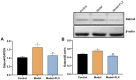
Premenstrual dysphoric disorder (PMDD), a form of premenstrual syndrome (PMS), is a severe health disturbance that affects a patient's emotions; it is caused by periodic psychological symptoms, and its pathogenesis remains unclear. As depression-like symptoms are found in a majority of clinical cases, a reliable animal model of premenstrual depression is indispensable to understand the pathogenesis. Herein, we describe a novel rat model of premenstrual depression, based on the forced swimming test, with a regular estrous cycle. The results showed that in the estrous cycle, the depression-like behavior of rats occurred in the non-receptive phase and disappeared in the receptive phase. Following ovariectomy, the depression-like symptoms disappeared and returned after a hormone priming regimen. Moreover, fluoxetine, an anti-depressant, could reverse the behavioral symptoms in these model rats with normal estrous cycle. Further, the model rats showed significant changes in the serum levels of estrogen and progesterone, hippocampal levels of allopregnanolone, 5-hydroxytryptamine, norepinephrine, and γ-aminobutyric acid (GABA), and in the expression of GABAA receptor 4α subunit, all of which were reversed to physiological levels by fluoxetine. Overall, we established a reliable and standardized rat model of premenstrual depression, which may facilitate the elucidation of PMS/PMDD pathogenesis and development of related therapies.
Keywords: Gabra4; animal model standardization; estrus cycle; forced swimming test; premenstrual dysphoric disorder.
Conflict of interest statement
Figures

References
-
- Eisenlohr-Moul TA, Kaiser G, Weise C, Schmalenberger KM, Kiesner J, Ditzen B, Kleinstäuber M. Are there temporal subtypes of premenstrual dysphoric disorder?: using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020; 50:964–72. 10.1017/S0033291719000849 - DOI - PMC - PubMed
-
- Yakir M, Klein-Laansma CT, Kreitler S, Brzezinski A, Oberbaum M, Vithoulkas G, Bentwich Z. A placebo-controlled double-blind randomized trial with individualized homeopathic treatment using a symptom cluster approach in women with premenstrual syndrome. Homeopathy. 2019; 108:256–69. 10.1055/s-0039-1691834 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

