Pannexin 1, a large-pore membrane channel, contributes to hypotonicity-induced ATP release in Schwann cells
- PMID: 33229726
- PMCID: PMC8178772
- DOI: 10.4103/1673-5374.290911
Pannexin 1, a large-pore membrane channel, contributes to hypotonicity-induced ATP release in Schwann cells
Abstract
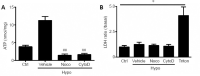
Pannexin 1 (Panx 1), as a large-pore membrane channel, is highly permeable to ATP and other signaling molecules. Previous studies have demonstrated the expression of Panx 1 in the nervous system, including astrocytes, microglia, and neurons. However, the distribution and function of Panx 1 in the peripheral nervous system are not clear. Blocking the function of Panx 1 pharmacologically (carbenoxolone and probenecid) or with small interfering RNA targeting pannexins can greatly reduce hypotonicity-induced ATP release. Treatment of Schwann cells with a Ras homolog family member (Rho) GTPase inhibitor and small interfering RNA targeting Rho or cytoskeleton disrupting agents, such as nocodazole or cytochalasin D, revealed that hypotonicity-induced ATP release depended on intracellular RhoA and the cytoskeleton. These findings suggest that Panx 1 participates in ATP release in Schwann cells by regulating RhoA and the cytoskeleton arrangement. This study was approved by the Animal Ethics Committee of Nantong University, China (No. S20180806-002) on August 5, 2018.
Keywords: ATP; Ras homolog family member A; Schwann cells; cytoskeleton; injury; neuron; pannexin 1; peripheral nerve.
Conflict of interest statement
None
Figures




References
-
- Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–716. - PubMed
-
- Begum G, Song S, Wang S, Zhao H, Bhuiyan MIH, Li E, Nepomuceno R, Ye Q, Sun M, Calderon MJ, Stolz DB, St Croix C, Watkins SC, Chen Y, He P, Shull GE, Sun D. Selective knockout of astrocytic Na(+) /H(+) exchanger isoform 1 reduces astrogliosis, BBB damage, infarction, and improves neurological function after ischemic stroke. Glia. 2018;66:126–144. - PMC - PubMed
-
- Boyce AK, Kim MS, Wicki-Stordeur LE, Swayne LA. ATP stimulates pannexin 1 internalization to endosomal compartments. Biochem J. 2015;470:319–330. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

