Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation
- PMID: 33230326
- PMCID: PMC7855371
- DOI: 10.1038/s41563-020-00853-9
Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation
Abstract
Directed differentiation of human pluripotent stem cells to kidney organoids brings the prospect of drug screening, disease modelling and the generation of tissue for renal replacement. Currently, these applications are hampered by organoid variability, nephron immaturity, low throughput and limited scale. Here, we apply extrusion-based three-dimensional cellular bioprinting to deliver rapid and high-throughput generation of kidney organoids with highly reproducible cell number and viability. We demonstrate that manual organoid generation can be replaced by 6- or 96-well organoid bioprinting and evaluate the relative toxicity of aminoglycosides as a proof of concept for drug testing. In addition, three-dimensional bioprinting enables precise manipulation of biophysical properties, including organoid size, cell number and conformation, with modification of organoid conformation substantially increasing nephron yield per starting cell number. This facilitates the manufacture of uniformly patterned kidney tissue sheets with functional proximal tubular segments. Hence, automated extrusion-based bioprinting for kidney organoid production delivers improvements in throughput, quality control, scale and structure, facilitating in vitro and in vivo applications of stem cell-derived human kidney tissue.
Figures

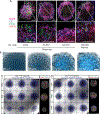
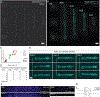



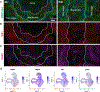







Comment in
-
Bioprinting better kidney organoids.Nat Mater. 2021 Feb;20(2):128-130. doi: 10.1038/s41563-020-00881-5. Nat Mater. 2021. PMID: 33504983 No abstract available.
References
Methods references
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

