miR-27a-3p Targets ATF3 to Reduce Calcium Deposition in Vascular Smooth Muscle Cells
- PMID: 33230462
- PMCID: PMC7578555
- DOI: 10.1016/j.omtn.2020.09.030
miR-27a-3p Targets ATF3 to Reduce Calcium Deposition in Vascular Smooth Muscle Cells
Abstract
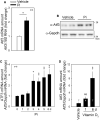
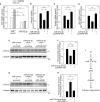
Vascular calcification, the ectopic deposition of calcium in blood vessels, develops in association with various metabolic diseases and atherosclerosis and is an independent predictor of morbidity and mortality associated with these diseases. Herein, we report that reduction of microRNA-27a-3p (miR-27a-3p) causes an increase in activating transcription factor 3 (ATF3), a novel osteogenic transcription factor, in vascular smooth muscle cells. Both microRNA (miRNA) and mRNA microarrays were performed with rat vascular smooth muscle cells, and reciprocally regulated pairs of miRNA and mRNA were selected after bioinformatics analysis. Inorganic phosphate significantly reduced the expression of miR-27a-3p in A10 cells. The transcript level was also reduced in vitamin D3-administered mouse aortas. miR-27a-3p mimic reduced calcium deposition, whereas miR-27a-3p inhibitor increased it. The Atf3 mRNA level was upregulated in a cellular vascular calcification model, and miR-27a-3p reduced the Atf3 mRNA and protein levels. Transfection with Atf3 could recover the miR-27a-3p-induced reduction of calcium deposition. Our results suggest that reduction of miR-27a-3p may contribute to the development of vascular calcification by de-repression of ATF3.
Keywords: activating transcription factor 3; miR-27a-3p; miRNA; vascular calcification; vascular smooth muscle cells.
© 2020 The Author(s).
Figures






Similar articles
-
MiRNA-27a-3p promotes osteogenic differentiation of human mesenchymal stem cells through targeting ATF3.Eur Rev Med Pharmacol Sci. 2019 Aug;23(3 Suppl):73-80. doi: 10.26355/eurrev_201908_18632. Eur Rev Med Pharmacol Sci. 2019. PMID: 31389577
-
Expression patterns of serum miR-27a-3p and activating transcription factor 3 in children with bronchial asthma and their correlations with airway inflammation.Clin Respir J. 2025 Mar;19(3):e13631. doi: 10.1111/crj.13631. Epub 2023 Jun 29. Clin Respir J. 2025. PMID: 37385291 Free PMC article.
-
Downregulation of miR-542-3p promotes osteogenic transition of vascular smooth muscle cells in the aging rat by targeting BMP7.Hum Genomics. 2019 Dec 11;13(1):67. doi: 10.1186/s40246-019-0245-z. Hum Genomics. 2019. PMID: 31829291 Free PMC article.
-
miR-27a-3p regulates proliferation and apoptosis of colon cancer cells by potentially targeting BTG1.Oncol Lett. 2019 Sep;18(3):2825-2834. doi: 10.3892/ol.2019.10629. Epub 2019 Jul 18. Oncol Lett. 2019. PMID: 31452761 Free PMC article.
-
ATF3 and its emerging role in atherosclerosis: a narrative review.Cardiovasc Diagn Ther. 2022 Dec;12(6):926-942. doi: 10.21037/cdt-22-206. Cardiovasc Diagn Ther. 2022. PMID: 36605071 Free PMC article. Review.
Cited by
-
MicroRNA-145 and microRNA-486 are potential serum biomarkers for vascular calcification.Nephrol Dial Transplant. 2023 Jun 30;38(7):1729-1740. doi: 10.1093/ndt/gfad027. Nephrol Dial Transplant. 2023. PMID: 36722155 Free PMC article.
-
Exploring the role of circ-GALK2 in vascular smooth muscle cell calcification: mechanisms and implications.Eur J Med Res. 2025 Aug 18;30(1):755. doi: 10.1186/s40001-025-03040-1. Eur J Med Res. 2025. PMID: 40826429 Free PMC article.
-
Identification of Molecular Signatures and Candidate Drugs in Vascular Dementia by Bioinformatics Analyses.Front Mol Neurosci. 2022 Feb 11;15:751044. doi: 10.3389/fnmol.2022.751044. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35221911 Free PMC article.
-
Integrated analysis of C3AR1 and CD163 associated with immune infiltration in intracranial aneurysms pathogenesis.Heliyon. 2023 Mar 11;9(3):e14470. doi: 10.1016/j.heliyon.2023.e14470. eCollection 2023 Mar. Heliyon. 2023. PMID: 36942257 Free PMC article.
-
Mechanisms Driving Palmitate-Mediated Neuronal Dysregulation in the Hypothalamus.Cells. 2021 Nov 11;10(11):3120. doi: 10.3390/cells10113120. Cells. 2021. PMID: 34831343 Free PMC article. Review.
References
-
- Chow B., Rabkin S.W. The relationship between arterial stiffness and heart failure with preserved ejection fraction: a systemic meta-analysis. Heart Fail. Rev. 2015;20:291–303. - PubMed
-
- Giachelli C.M., Jono S., Shioi A., Nishizawa Y., Mori K., Morii H. Vascular calcification and inorganic phosphate. Am. J. Kidney Dis. 2001;38(4, Suppl 1):S34–S37. - PubMed
-
- Abedin M., Tintut Y., Demer L.L. Vascular calcification: mechanisms and clinical ramifications. Arterioscler. Thromb. Vasc. Biol. 2004;24:1161–1170. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

