Use of Productivity Loss/Gain in Cost-Effectiveness Analyses for Drugs: A Systematic Review
- PMID: 33230613
- PMCID: PMC7790765
- DOI: 10.1007/s40273-020-00986-4
Use of Productivity Loss/Gain in Cost-Effectiveness Analyses for Drugs: A Systematic Review
Abstract
Background: Inclusion of productivity losses and gains in cost-effectiveness analyses for drugs is recommended by pharmacoeconomic guidelines in some countries and is considered optional in others. Often guidelines recommend analysis based on the payer perspective, but suggest that a supplemental analysis based on the societal perspective may be submitted that includes productivity losses/gains. However, there is no universally recognized framework for the approach to including productivity losses and gains in pharmacoeconomic analyses.
Objectives: This study aimed to systematically review literature on cost-effectiveness analyses of drug interventions that included costs associated with productivity losses/gains and to summarize the types cost elements included and cost calculation methods employed. Moreover, this study examines variations in the calculation of productivity losses/gains by target disease type, geographic region, income group, period of analysis, and analysis time horizon-as well as the impact of their inclusion on the study outcomes.
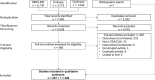
Methods: A search of three databases was performed, including MEDLINE, Embase, and the Cochrane Library, to identify cost-effectiveness and cost-utility analyses that included indirect costs such as productivity losses/gains. Publications from January 2010 to October 2019 were examined and selected for inclusion by two independent reviewers. In addition to the citation details, data on the country of analysis, country income group, target disease area, study sponsorship, type of analysis, study design, time horizon, analysis perspective, productivity loss/gain elements included, the approach used to estimate productivity losses/gains, and the impact of their inclusion on the study outcome-namely the incremental cost effectiveness ratio-were extracted and summarized.
Results: The search strategy identified 5038 unique studies, and 208 were included in the final analysis. Among the studies reviewed, 165 (79%) were conducted in high-income countries and 160 (77%) were conducted for North American and European/Central Asian countries. The productivity loss/gain elements included in the analysis were reported for 169 studies (81%). Absenteeism only was included for 98 studies (47%), and absenteeism plus presenteeism was included for 29 studies (14%). Absenteeism plus some other element such as costs associated with unemployment and/or early retirement was included for 32 studies (15%) examined. Only one out of four of the studies reviewed included information on the approach used to estimate productivity losses/gains, which was predominantly the human capital approach. One-hundred forty-four studies (69%) reported the impact of including productivity losses/gains on the ICER, with 110 studies (53%) reporting that their inclusion contributed to more favorable cost-effectiveness.
Conclusions: Although inclusion of productivity losses/gains was shown to have a favorable impact on evaluations for many studies, their impact and method of calculation was often not reported or was unclear. Further examination and discussion is needed to consider the optimal framework for considering productivity losses/gains in cost-effectiveness analyses, including the appropriate cost elements to include (e.g., patient absenteeism, caregiver absenteeism, presenteeism, unemployment, etc.) and how those costs should be estimated.
Conflict of interest statement
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Akira Yuasa and Naohiro Yonemoto are full-time employees of Pfizer Japan Inc. Michael LoPresti is a full-time employee of INTAGE Healthcare Inc. Shunya Ikeda declares no conficts of interest associated with this manuscript.
Figures
Similar articles
-
Productivity loss/gain in cost-effectiveness analyses for vaccines: a systematic review.Expert Rev Pharmacoecon Outcomes Res. 2021 Apr;21(2):235-245. doi: 10.1080/14737167.2021.1881484. Epub 2021 Feb 23. Expert Rev Pharmacoecon Outcomes Res. 2021. PMID: 33593223
-
A Systematic Review of Productivity in Economic Evaluations of Workplace Interventions: A Need for Reporting Criteria?Appl Health Econ Health Policy. 2019 Oct;17(5):591-613. doi: 10.1007/s40258-019-00473-8. Appl Health Econ Health Policy. 2019. PMID: 30937837
-
Lost productivity in four European countries among patients with rheumatic disorders: are absenteeism and presenteeism transferable?Pharmacoeconomics. 2012 Sep 1;30(9):795-807. doi: 10.2165/11591520-000000000-00000. Pharmacoeconomics. 2012. PMID: 22670593
-
The Impact of Inflammatory Bowel Disease in Canada 2018: Indirect Costs of IBD Care.J Can Assoc Gastroenterol. 2019 Feb;2(Suppl 1):S34-S41. doi: 10.1093/jcag/gwy050. Epub 2018 Nov 2. J Can Assoc Gastroenterol. 2019. PMID: 31294383 Free PMC article.
-
Public sector reforms and their impact on the level of corruption: A systematic review.Campbell Syst Rev. 2021 May 24;17(2):e1173. doi: 10.1002/cl2.1173. eCollection 2021 Jun. Campbell Syst Rev. 2021. PMID: 37131927 Free PMC article. Review.
Cited by
-
Incorporating productivity loss in health economic evaluations: a review of guidelines and practices worldwide for research agenda in China.BMJ Glob Health. 2022 Aug;7(8):e009777. doi: 10.1136/bmjgh-2022-009777. BMJ Glob Health. 2022. PMID: 35977755 Free PMC article. Review.
-
The Primary Process and Key Concepts of Economic Evaluation in Healthcare.J Prev Med Public Health. 2022 Sep;55(5):415-423. doi: 10.3961/jpmph.22.195. Epub 2022 Aug 24. J Prev Med Public Health. 2022. PMID: 36229903 Free PMC article.
-
Improvement in quality of life after asfotase alfa treatment in adults with pediatric-onset hypophosphatasia: data from 5 patient-reported outcome measures.JBMR Plus. 2024 May 7;8(8):ziae062. doi: 10.1093/jbmrpl/ziae062. eCollection 2024 Aug. JBMR Plus. 2024. PMID: 39006866 Free PMC article.
-
Cost-benefit analysis of haemodialysis in patients with end-stage kidney disease in Abuja, Nigeria.Health Econ Rev. 2024 Jul 3;14(1):47. doi: 10.1186/s13561-024-00529-z. Health Econ Rev. 2024. PMID: 38958775 Free PMC article.
-
Socioeconomic and fiscal returns of expanded investment in immunization: a case for life-course vaccination in Colombia.Health Aff Sch. 2024 Apr 25;2(4):qxae042. doi: 10.1093/haschl/qxae042. eCollection 2024 Apr. Health Aff Sch. 2024. PMID: 38756168 Free PMC article.
References
-
- Neumann PJ, Russell LB, Siegel JE, Prosser LA, Krahn M, Mandelblatt JS, et al. Using cost-effectiveness in health and medicine: experiences since the original panel. In: Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG, et al., editors. Cost-effectiveness in health and medicine. New York: Oxford University Press; 2017. pp. 1–38.
-
- International Society for Pharmacoeconomics and Outcomes Research I, (ISPOR). Pharmacoeconomic guidelines around the world. International Society for Pharmacoeconomics and Outcomes Research, Lawrenceville, NJ, USA. 2020. https://www.tools.ispor.org/peguidelines/. Accessed 29 Mar 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical