The ADAMTS13-von Willebrand factor axis in COVID-19 patients
- PMID: 33230904
- PMCID: PMC7753796
- DOI: 10.1111/jth.15191
The ADAMTS13-von Willebrand factor axis in COVID-19 patients
Abstract
Background: Severe coronavirus disease 2019 (COVID-19) is characterized by an increased risk of thromboembolic events, with evidence of microthrombosis in the lungs of deceased patients.
Objectives: To investigate the mechanism of microthrombosis in COVID-19 progression.
Patients/methods: We assessed von Willebrand factor (VWF) antigen (VWF:Ag), VWF ristocetin-cofactor (VWF:RCo), VWF multimers, VWF propeptide (VWFpp), and ADAMTS13 activity in a cross-sectional study of 50 patients stratified according to their admission to three different intensity of care units: low (requiring high-flow nasal cannula oxygenation, n = 14), intermediate (requiring continuous positive airway pressure devices, n = 17), and high (requiring mechanical ventilation, n = 19).
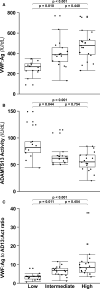
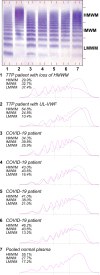
Results: Median VWF:Ag, VWF:RCo, and VWFpp levels were markedly elevated in COVID-19 patients and increased with intensity of care, with VWF:Ag being 268, 386, and 476 IU/dL; VWF:RCo 216, 334, and 388 IU/dL; and VWFpp 156, 172, and 192 IU/dL in patients at low, intermediate, and high intensity of care, respectively. Conversely, the high-to-low molecular-weight VWF multimers ratios progressively decreased with increasing intensity of care, as well as median ADAMTS13 activity levels, which ranged from 82 IU/dL for patients at low intensity of care to 62 and 55 IU/dL for those at intermediate and high intensity of care.
Conclusions: We found a significant alteration of the VWF-ADAMTS13 axis in COVID-19 patients, with an elevated VWF:Ag to ADAMTS13 activity ratio that was strongly associated with disease severity. Such an imbalance enhances the hypercoagulable state of COVID-19 patients and their risk of microthrombosis.
Keywords: ADAMTS13 Protein; COVID-19; Microvasculature; Severe acute respiratory syndrome coronavirus 2; Thrombosis; von Willebrand factor.
© 2020 International Society on Thrombosis and Haemostasis.
Figures
Comment in
-
Thrombotic thrombocytopenic purpura as the initial presentation of COVID-19.J Thromb Haemost. 2021 Apr;19(4):1132-1134. doi: 10.1111/jth.15231. J Thromb Haemost. 2021. PMID: 33382912 Free PMC article. No abstract available.
-
Thrombotic thrombocytopenic purpura (TTP) response following COVID-19 infection: Implications for the ADAMTS-13-von Willebrand factor axis.J Thromb Haemost. 2021 Apr;19(4):1130-1132. doi: 10.1111/jth.15230. J Thromb Haemost. 2021. PMID: 33382919 Free PMC article. No abstract available.
Similar articles
-
Von Willebrand factor collagen-binding capacity predicts in-hospital mortality in COVID-19 patients: insight from VWF/ADAMTS13 ratio imbalance.Angiogenesis. 2021 Aug;24(3):407-411. doi: 10.1007/s10456-021-09789-3. Epub 2021 May 11. Angiogenesis. 2021. PMID: 33974165 Free PMC article.
-
ADAMTS13 activity to von Willebrand factor antigen ratio predicts acute kidney injury in patients with COVID-19: Evidence of SARS-CoV-2 induced secondary thrombotic microangiopathy.Int J Lab Hematol. 2021 Jul;43 Suppl 1(Suppl 1):129-136. doi: 10.1111/ijlh.13415. Epub 2020 Dec 3. Int J Lab Hematol. 2021. PMID: 33270980 Free PMC article.
-
Levels and activities of von Willebrand factor and metalloproteinase with thrombospondin type-1 motif, number 13 in inflammatory bowel diseases.World J Gastroenterol. 2017 Jul 14;23(26):4796-4805. doi: 10.3748/wjg.v23.i26.4796. World J Gastroenterol. 2017. PMID: 28765701 Free PMC article.
-
Increased VWF and Decreased ADAMTS-13 in COVID-19: Creating a Milieu for (Micro)Thrombosis.Semin Thromb Hemost. 2021 Jun;47(4):400-418. doi: 10.1055/s-0041-1727282. Epub 2021 Apr 23. Semin Thromb Hemost. 2021. PMID: 33893632 Review.
-
Contribution of the von Willebrand factor/ADAMTS13 imbalance to COVID-19 coagulopathy.Am J Physiol Heart Circ Physiol. 2022 Jan 1;322(1):H87-H93. doi: 10.1152/ajpheart.00204.2021. Epub 2021 Dec 10. Am J Physiol Heart Circ Physiol. 2022. PMID: 34890277 Free PMC article. Review.
Cited by
-
A randomised controlled trial of plasma exchange compared to standard of care in the treatment of severe COVID-19 infection (COVIPLEX).Sci Rep. 2024 Jul 23;14(1):16876. doi: 10.1038/s41598-024-67028-3. Sci Rep. 2024. PMID: 39043682 Free PMC article. Clinical Trial.
-
Reply.Arthritis Rheumatol. 2022 Sep;74(9):1603-1604. doi: 10.1002/art.42141. Epub 2022 Jul 21. Arthritis Rheumatol. 2022. PMID: 35436032 Free PMC article. No abstract available.
-
Vascular risk factors for COVID-19 ARDS: endothelium, contact-kinin system.Front Med (Lausanne). 2023 Jun 28;10:1208866. doi: 10.3389/fmed.2023.1208866. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37448794 Free PMC article. Review.
-
COVID-19 coagulopathies: Human blood proteins mimic SARS-CoV-2 virus, vaccine proteins and bacterial co-infections inducing autoimmunity: Combinations of bacteria and SARS-CoV-2 synergize to induce autoantibodies targeting cardiolipin, cardiolipin-binding proteins, platelet factor 4, prothrombin, and coagulation factors.Bioessays. 2021 Dec;43(12):e2100158. doi: 10.1002/bies.202100158. Epub 2021 Oct 22. Bioessays. 2021. PMID: 34677872 Free PMC article.
-
COVID-19 infection triggering Thrombotic Thrombocytopenic Purpura.IDCases. 2021;26:e01256. doi: 10.1016/j.idcr.2021.e01256. Epub 2021 Aug 24. IDCases. 2021. PMID: 34458098 Free PMC article.
References
-
- WHO. Who weekly epidemiological update – 20 October 2020. Data as received by WHO from national authorities, as of 18 October 2020, 10 am CEST, 2020. https://www.who.int/publications/m/item/weekly‐epidemiological‐update–‐2.... Accessed October 24, 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

