Development, performance evaluation, and clinical application of a Rapid SARS-CoV-2 IgM and IgG Test Kit based on automated fluorescence immunoassay
- PMID: 33231312
- PMCID: PMC7753814
- DOI: 10.1002/jmv.26696
Development, performance evaluation, and clinical application of a Rapid SARS-CoV-2 IgM and IgG Test Kit based on automated fluorescence immunoassay
Abstract
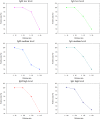
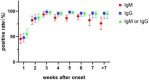
The ongoing coronavirus disease 2019 (COVID-19) epidemic has made a huge impact on health, economies, and societies all over the world. Although reverse transcription-polymerase chain reaction (RT-PCR)-based nucleic acid detection has been primarily used in the diagnosis of COVID-19, it is time-consuming with limited application scenarios and must be operated by qualified personnel. Antibody test, particularly point-of-care antibody testing, is a suitable complement to nucleic acid test as it provides rapid, portable, and cost-effective detection of infections. In this study, a Rapid Antibody Test Kit was developed based on fluorescence immunochromatography for the sensitive, accurate, and automated detection of immunoglobulin M (IgM) and IgG antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human serum, plasma, and whole blood samples within 10 min. The sensitivity, specificity, precision, and stability of the test kit were of good performance. No cross-activity and no interference was observed. In the multiple-center parallel study, 223 samples from hospitalized patients were used to evaluate the clinical specificity of the test. Both SARS-CoV-2 IgM and IgG achieved a clinical specificity of 98.21%. The clinical sensitivities of SARS-CoV-2 IgM and IgG were 79.54% and 87.45%, respectively, among 733 reverse transcription-polymerase chain reaction (RT-PCR) confirmed SARS-CoV-2 samples. For the combined IgM and IgG assays, the sensitivity and specificity were 89.22% and 96.86%, respectively. Our results demonstrate that the combined use of IgM and IgG could serve as a more suitable alternative detection method for patients with COVID-19, and the developed kit is of great public health significance for the prevention and control of the COVID-19 pandemic.
Keywords: COVID-19; IgM and IgG; SARS-CoV-2; automated detection; fluorescence immunochromatography; rapid antibody test.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures

Similar articles
-
Clinical diagnostic performance evaluation of five immunoassays for antibodies to SARS-CoV-2 diagnosis in a real-life routine care setting.Pan Afr Med J. 2021 May 3;39:3. doi: 10.11604/pamj.2021.39.3.26471. eCollection 2021. Pan Afr Med J. 2021. PMID: 34178231 Free PMC article.
-
Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis.J Med Virol. 2020 Sep;92(9):1518-1524. doi: 10.1002/jmv.25727. Epub 2020 Apr 13. J Med Virol. 2020. PMID: 32104917 Free PMC article.
-
Clinical validation of a second generation anti-SARS-CoV-2 IgG and IgM automated chemiluminescent immunoassay.J Med Virol. 2021 Apr;93(4):2523-2528. doi: 10.1002/jmv.26809. Epub 2021 Jan 26. J Med Virol. 2021. PMID: 33463719 Free PMC article.
-
Clinical applications of detecting IgG, IgM or IgA antibody for the diagnosis of COVID-19: A meta-analysis and systematic review.Int J Infect Dis. 2021 Mar;104:415-422. doi: 10.1016/j.ijid.2021.01.016. Epub 2021 Jan 12. Int J Infect Dis. 2021. PMID: 33450372 Free PMC article.
-
Serological tests for COVID-19: Potential opportunities.Cell Biol Int. 2021 Apr;45(4):740-748. doi: 10.1002/cbin.11516. Epub 2021 Jan 13. Cell Biol Int. 2021. PMID: 33289157 Free PMC article. Review.
Cited by
-
Development of practical techniques for simultaneous detection and distinction of current and emerging SARS-CoV-2 variants.Anal Sci. 2023 Nov;39(11):1839-1856. doi: 10.1007/s44211-023-00396-4. Epub 2023 Jul 30. Anal Sci. 2023. PMID: 37517003 Review.
-
A systematic review and meta-analysis of the sensitivity of antibody tests for the laboratory confirmation of COVID-19.Future Virol. 2021 Nov:10.2217/fvl-2021-0211. doi: 10.2217/fvl-2021-0211. Epub 2021 Dec 15. Future Virol. 2021. PMID: 34950219 Free PMC article. Review.
-
Anti-SARS-CoV-2 antibody response in patients with chronic lymphocytic leukemia.Leukemia. 2020 Nov;34(11):3047-3049. doi: 10.1038/s41375-020-01030-2. Epub 2020 Aug 27. Leukemia. 2020. PMID: 32855439 Free PMC article. No abstract available.
-
Rapid detection of SARS-CoV-2: The gradual boom of lateral flow immunoassay.Front Bioeng Biotechnol. 2023 Jan 10;10:1090281. doi: 10.3389/fbioe.2022.1090281. eCollection 2022. Front Bioeng Biotechnol. 2023. PMID: 36704307 Free PMC article. Review.
-
Recent Advances in Lateral Flow Assays for Viral Protein Detection with Nanomaterial-Based Optical Sensors.Biosensors (Basel). 2024 Apr 17;14(4):197. doi: 10.3390/bios14040197. Biosensors (Basel). 2024. PMID: 38667190 Free PMC article. Review.
References
-
- World Health Organization . Coronavirus disease (COVID‐19) situation report‐198. Geneva, Switzerland: World Health Organization; 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

