Prefusion spike protein stabilization through computational mutagenesis
- PMID: 33231324
- PMCID: PMC7753443
- DOI: 10.1002/prot.26025
Prefusion spike protein stabilization through computational mutagenesis
Abstract
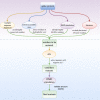
A novel severe acute respiratory syndrome (SARS)-like coronavirus (SARS-CoV-2) has emerged as a human pathogen, causing global pandemic and resulting in over 400 000 deaths worldwide. The surface spike protein of SARS-CoV-2 mediates the process of coronavirus entry into human cells by binding angiotensin-converting enzyme 2 (ACE2). Due to the critical role in viral-host interaction and the exposure of spike protein, it has been a focus of most vaccines' developments. However, the structural and biochemical studies of the spike protein are challenging because it is thermodynamically metastable. Here, we develop a new pipeline that automatically identifies mutants that thermodynamically stabilize the spike protein. Our pipeline integrates bioinformatics analysis of conserved residues, motion dynamics from molecular dynamics simulations, and other structural analysis to identify residues that significantly contribute to the thermodynamic stability of the spike protein. We then utilize our previously developed protein design tool, Eris, to predict thermodynamically stabilizing mutations in proteins. We validate the ability of our pipeline to identify protein stabilization mutants through known prefusion spike protein mutants. We finally utilize the pipeline to identify new prefusion spike protein stabilization mutants.
Keywords: computational mutagenesis; coronavirus; protein stabilization; spike protein.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures

Similar articles
-
Molecular dynamic simulation analysis of SARS-CoV-2 spike mutations and evaluation of ACE2 from pets and wild animals for infection risk.Comput Biol Chem. 2022 Feb;96:107613. doi: 10.1016/j.compbiolchem.2021.107613. Epub 2021 Dec 1. Comput Biol Chem. 2022. PMID: 34896769 Free PMC article.
-
Computational Alanine Scanning and Structural Analysis of the SARS-CoV-2 Spike Protein/Angiotensin-Converting Enzyme 2 Complex.ACS Nano. 2020 Sep 22;14(9):11821-11830. doi: 10.1021/acsnano.0c04674. Epub 2020 Aug 26. ACS Nano. 2020. PMID: 32833435 Free PMC article.
-
Static all-atom energetic mappings of the SARS-Cov-2 spike protein and dynamic stability analysis of "Up" versus "Down" protomer states.PLoS One. 2020 Nov 10;15(11):e0241168. doi: 10.1371/journal.pone.0241168. eCollection 2020. PLoS One. 2020. PMID: 33170884 Free PMC article.
-
Structural basis of severe acute respiratory syndrome coronavirus 2 infection.Curr Opin HIV AIDS. 2021 Jan;16(1):74-81. doi: 10.1097/COH.0000000000000658. Curr Opin HIV AIDS. 2021. PMID: 33186231 Review.
-
Molecular Dynamics Studies on the Structural Characteristics for the Stability Prediction of SARS-CoV-2.Int J Mol Sci. 2021 Aug 13;22(16):8714. doi: 10.3390/ijms22168714. Int J Mol Sci. 2021. PMID: 34445414 Free PMC article. Review.
Cited by
-
Vaccine development and technology for SARS-CoV-2: Current insight.J Med Virol. 2022 Mar;94(3):878-896. doi: 10.1002/jmv.27425. Epub 2021 Nov 11. J Med Virol. 2022. PMID: 34713912 Free PMC article. Review.
-
SARS-CoV-2 spike protein as a bacterial lipopolysaccharide delivery system in an overzealous inflammatory cascade.J Mol Cell Biol. 2023 Feb 7;14(9):mjac058. doi: 10.1093/jmcb/mjac058. J Mol Cell Biol. 2023. PMID: 36240490 Free PMC article.
References
-
- Cascella, M. , Rajnik, M. , Cuomo, A. , Dulebohn, S. C. & Di Napoli, R. Features, evaluation and treatment coronavirus (COVID‐19). in Statpearls [internet] (StatPearls Publishing), 2020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

