A phase 2, randomized, double-blind, placebo-controlled trial of AMG 301, a pituitary adenylate cyclase-activating polypeptide PAC1 receptor monoclonal antibody for migraine prevention
- PMID: 33231489
- PMCID: PMC7786389
- DOI: 10.1177/0333102420970889
A phase 2, randomized, double-blind, placebo-controlled trial of AMG 301, a pituitary adenylate cyclase-activating polypeptide PAC1 receptor monoclonal antibody for migraine prevention
Abstract
Objective: To assess the safety and efficacy of AMG 301, an inhibitor of the pituitary adenylate cyclase-activating polypeptide (PACAP)-1 (PAC1) receptor, for prevention of migraine.
Methods: In a double-blind trial, patients were randomized 4:3:3 to placebo, AMG 301 210 mg every 4 weeks, or AMG 301 420 mg every 2 weeks for 12 weeks. Effect on monthly migraine days and other secondary measures were assessed over weeks 9-12. Safety and tolerability were assessed.
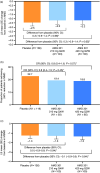
Results: Of 343 randomized patients (mean age, 41.8-42.5 years), the majority were women (85.4-90.4%), white (94.1-96.2%), and had episodic migraine (62.5-67.9%). A total of 305 patients completed treatment (placebo, n = 124; AMG 301 210 mg, n = 94; AMG 301 420 mg, n = 87). Least squares mean reduction at week 12 in monthly migraine days from baseline was -2.5 (0.4) days for placebo and -2.2 (0.5) days for both AMG 301 treatment groups. No difference between AMG 301 and placebo on any measure of efficacy was observed; mean (95% confidence interval) treatment difference versus placebo for monthly migraine days for AMG 301 210 mg, 0.3 (-0.9 to 1.4); AMG 301 420 mg, 0.3 (-0.9 to 1.4). The incidence of adverse events was similar across groups.
Conclusion: AMG 301 offered no benefit over placebo for migraine prevention; further studies may be necessary to fully understand the role of PACAP isoforms and its receptors in migraine pathophysiology.
Study registration: ClinicalTrials.gov: NCT03238781.
Keywords: AMG 301; chronic migraine; episodic migraine; pituitary adenylate cyclase-activating polypeptide; preventive treatment.
Conflict of interest statement
DD is a speaker, consultant or scientific advisor for Allergan, Amgen, Eli Lilly, Novartis, and Teva, as well as a primary investigator for Alder, Allergan, Amgen, Eli Lilly, Novartis, and Teva.
JHB reports no conflicts. JK is an employee of Novartis and owns Novartis stock. LZ, HP and DDM are employees of Amgen Inc., and own Amgen stock/stock options.
Figures



References
-
- Hargreaves R, Olesen J. Calcitonin gene-related peptide modulators – the history and renaissance of a new migraine drug class. Headache 2019; 59: 951–970. - PubMed
-
- Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: A new era in migraine therapy. Lancet 2019; 394: 1765–1774. - PubMed
-
- Dodick DW. CGRP ligand and receptor monoclonal antibodies for migraine prevention: Evidence review and clinical implications. Cephalalgia 2019; 39: 445–458. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

