Sesn2 attenuates the damage of endothelial progenitor cells induced by angiotensin II through regulating the Keap1/Nrf2 signal pathway
- PMID: 33231566
- PMCID: PMC7803511
- DOI: 10.18632/aging.104156
Sesn2 attenuates the damage of endothelial progenitor cells induced by angiotensin II through regulating the Keap1/Nrf2 signal pathway
Abstract
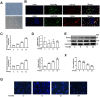
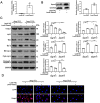
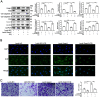
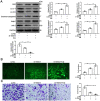
Endothelial progenitor cell (EPC) dysfunction is an important physiopathological mechanism in the dynamics of the formation of atherosclerosis. It has been reported that angiotensin II (Ang-II) damages the function of EPCs in atherosclerotic plaque through induction of oxidative stress. Sestrin 2 (Sesn2) serves as an antioxidant role in oxidative stress, however, the exact mechanisms underlying the dynamics of how Sesn2 may factor into EPCs after Ang-II treatments needs to be illustrated. We isolated EPCs from human umbilical cord blood samples and treated with Ang-II. Western blotting, qRT-PCR, transwell assays, immunofluorescence and so on were used to investigate the mechanisms underlying the roles of Sesn2 in EPCs treated with Ang-II. Ang-II was found to promote the apoptosis of EPCs as well as inhibited the mRNA and protein expression of Sesn2. Upregulation of Sesn2 attenuated the negative effect of Ang-II. Sesn2 increased the protein expression of Nrf2 by enhancing P62-dependent autophagy. Silencing of Nrf2 enhanced the degree of apoptosis of EPCs as well as resulted in the impairment of EPC functions through inducing the promotion of (reactive oxygen species) ROS production. Our study results indicated that Sesn2 facilitated the viability of EPCs After treatment with Ang-II, as well as provided a potential therapeutic target to alleviate the progression of atherosclerosis.
Keywords: Nrf2; angiotensins; atherosclerosis; endothelial progenitor cells; sestrin 2.
Conflict of interest statement
Figures



Similar articles
-
Sulodexide protects endothelial cells against 4-hydroxynonenal-induced oxidative stress and glutathione-dependent redox imbalance by modulation of sestrin2/nuclear factor erythroid 2-related factor 2 pathway.J Physiol Pharmacol. 2024 Aug;75(4). doi: 10.26402/jpp.2024.4.03. Epub 2024 Oct 10. J Physiol Pharmacol. 2024. PMID: 39415523
-
Sestrin2 overexpression alleviates hydrogen peroxide-induced apoptosis and oxidative stress in retinal ganglion cells by enhancing Nrf2 activation via Keap1 downregulation.Chem Biol Interact. 2020 Jun 1;324:109086. doi: 10.1016/j.cbi.2020.109086. Epub 2020 Apr 7. Chem Biol Interact. 2020. PMID: 32275923
-
Upregulation Sestrin2 protects against hydrogen peroxide-induced oxidative damage bovine mammary epithelial cells via a Keap1-Nrf2/ARE pathway.J Cell Physiol. 2021 Jan;236(1):392-404. doi: 10.1002/jcp.29867. Epub 2020 Jun 9. J Cell Physiol. 2021. PMID: 32519422
-
Novel target for treating Alzheimer's Diseases: Crosstalk between the Nrf2 pathway and autophagy.Ageing Res Rev. 2021 Jan;65:101207. doi: 10.1016/j.arr.2020.101207. Epub 2020 Nov 1. Ageing Res Rev. 2021. PMID: 33144123 Review.
-
Sestrin 2, a potential star of antioxidant stress in cardiovascular diseases.Free Radic Biol Med. 2021 Feb 1;163:56-68. doi: 10.1016/j.freeradbiomed.2020.11.015. Epub 2020 Dec 10. Free Radic Biol Med. 2021. PMID: 33310138 Review.
Cited by
-
Target Sestrin2 to Rescue the Damaged Organ: Mechanistic Insight into Its Function.Oxid Med Cell Longev. 2021 Nov 2;2021:8790369. doi: 10.1155/2021/8790369. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34765085 Free PMC article. Review.
-
NRF2 Activation and Downstream Effects: Focus on Parkinson's Disease and Brain Angiotensin.Antioxidants (Basel). 2021 Oct 20;10(11):1649. doi: 10.3390/antiox10111649. Antioxidants (Basel). 2021. PMID: 34829520 Free PMC article. Review.
-
Magnesium promotes vascularization and osseointegration in diabetic states.Int J Oral Sci. 2024 Jan 31;16(1):10. doi: 10.1038/s41368-023-00271-y. Int J Oral Sci. 2024. PMID: 38296940 Free PMC article.
-
Sestrin2 Signaling Pathway Regulates Podocyte Biology and Protects against Diabetic Nephropathy.J Diabetes Res. 2023 Feb 10;2023:8776878. doi: 10.1155/2023/8776878. eCollection 2023. J Diabetes Res. 2023. PMID: 36818747 Free PMC article. Review.
-
Sesn2 Serves as a Regulator between Mitochondrial Unfolded Protein Response and Mitophagy in Intervertebral Disc Degeneration.Int J Biol Sci. 2023 Jan 1;19(2):571-592. doi: 10.7150/ijbs.70211. eCollection 2023. Int J Biol Sci. 2023. PMID: 36632468 Free PMC article.
References
-
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, et al.. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012; 380:2224–60. 10.1016/S0140-6736(12)61766-8 - DOI - PMC - PubMed
-
- Giannotti G, Doerries C, Mocharla PS, Mueller MF, Bahlmann FH, Horvàth T, Jiang H, Sorrentino SA, Steenken N, Manes C, Marzilli M, Rudolph KL, Lüscher TF, et al.. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension. 2010; 55:1389–97. 10.1161/HYPERTENSIONAHA.109.141614 - DOI - PubMed
-
- Yang Z, Chen L, Su C, Xia WH, Wang Y, Wang JM, Chen F, Zhang YY, Wu F, Xu SY, Zhang XL, Tao J. Impaired endothelial progenitor cell activity is associated with reduced arterial elasticity in patients with essential hypertension. Clin Exp Hypertens. 2010; 32:444–52. 10.3109/10641961003686435 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

